40 compare and contrast heat and temperature
thermodynamics - What is the difference between heat and light ... 0. Heat and light are different but they are both forms of energy. Heat is a form of kinetic energy contained in the random motion of the particles of a material. Light is a form of electromagnetic energy. As with other forms of energy, heat energy can be transformed into light energy and vice versa. What are Heating and Cooling Curves? - Study.com Lesson Transcript. LaRita holds a master's degree and is currently an adjunct professor of Chemistry. Heating and cooling curves are graphs of temperature over time for different substances ...
Heat vs work - Energy Education The the distinction between Heat and Work is important in the field of thermodynamics. Heat is the transfer of thermal energy between systems, while work is the transfer of mechanical energy between two systems. ... Temperature difference Process: Macroscopic pushes and pulls: Microscopic collisions Positive value: W > 0 when a gas is ...
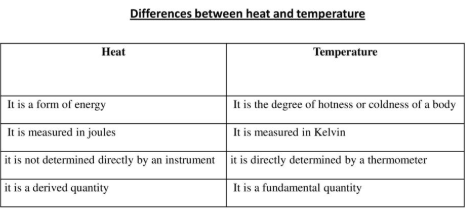
Compare and contrast heat and temperature
Heat vs. Temperature Comparison & Contrast | What is the Difference ... Though in casual usage they are often confused, heat and temperature are not the same. Heat measures the total energy, both kinetic and potential of the particles that make up some body.... 2.2: Energy, Heat, and Temperature - Chemistry LibreTexts As a body loses or gains heat, its temperature changes in direct proportion to the amount of thermal energy q transferred: (2.2.3) q = C Δ T. The proportionality constant C is known as the heat capacity. (2.2.4) C = q Δ T. If Δ T is expressed in kelvins (degrees) and q in joules, the units of C are J K -1. Chemistry Flashcards | Quizlet Compare and contrast heat and temperature. Heat is a form of energy and temperature is measurement of the energy that a material is releasing. In which direction does energy flow when you touch an ice cube? From your finger to the ice because heat flows from warm to cold, not cold to warm.
Compare and contrast heat and temperature. Heat, Temperature, and Thermal Energy Transfer - CK-12 Foundation Covers heat, temperature, and thermal energy. Click Create Assignment to assign this modality to your LMS. We have a new and improved read on this topic. Difference Between Heat And Temperature - VEDANTU Are Heat and Temperature the same? Heat Temperature Heat and Temperature: Heat is the energy stored inside an object. The coldness or hotness of an object is measured by temperature. An object's temperature cannot tell us how much heat energy it has. It's easy to see, but why not if you think about an iceberg and an ice cube. Heat Exhaustion vs. Heatstroke: What's the Difference? Heat cramps: Painful muscle cramps can strike when you're exercising in hot weather. They develop when you sweat so much that your body loses salts and fluids. Heat exhaustion: More serious than ... Global Warming vs. Climate Change Since the pre-industrial period, human activities are estimated to have increased Earth's global average temperature by about 1 degree Celsius (1.8 degrees Fahrenheit), a number that is currently increasing by more than 0.2 degrees Celsius (0.36 degrees Fahrenheit) per decade.
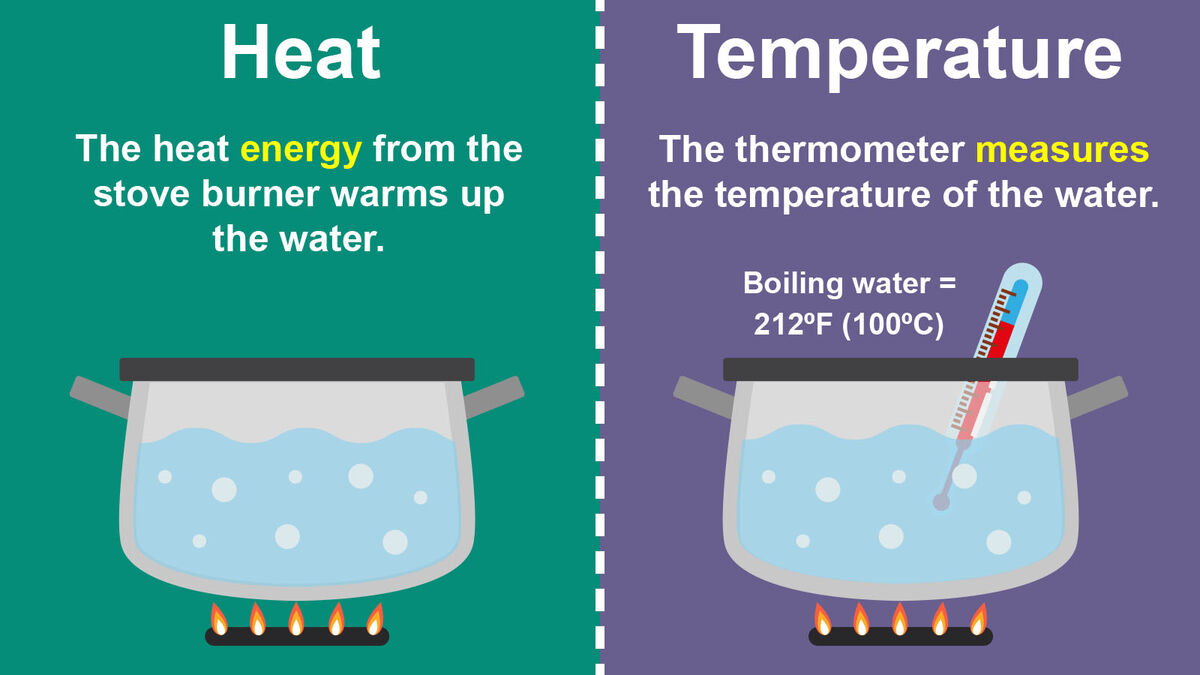
Heat vs Temperature: What are the Similarities ... - Sciencing Heat is the form of energy transferred between two materials that are at different temperatures. Heat always flows from the material with higher temperature to the material with lower temperature until thermal equilibrium is reached. Since heat is a form of energy, the SI unit of heat is the joule. Differences Between Heat and Temperature 11.1 Temperature and Thermal Energy - Physics | OpenStax Heat is the transfer of energy due to a temperature difference. Temperature is defined in terms of the instrument we use to tell us how hot or cold an object is, based on a mechanism and scale invented by people. Temperature is literally defined as what we measure on a thermometer. Heat is often confused with temperature. 1.11: Temperature, Heat, and Energy - Chemistry LibreTexts The key difference is that heat deals with the transfer of thermal energy, whereas … Heat and temperature are a closely related topics, although the difference between the two are often conflated. ... Figure \(\PageIndex{3}\): A Comparison of the Fahrenheit, Celsius, and Kelvin Temperature Scales. Because the difference between the freezing ... Heat vs temperature - Energy Education Heat is the transfer of thermal energy, whereas temperature is a property the object exhibits. [1] Whats the difference? Heat describes the transfer of thermal energy between molecules within a system and is measured in Joules. [2] Heat measures how energy moves or flows. An object can gain heat or lose heat, but it cannot have heat.
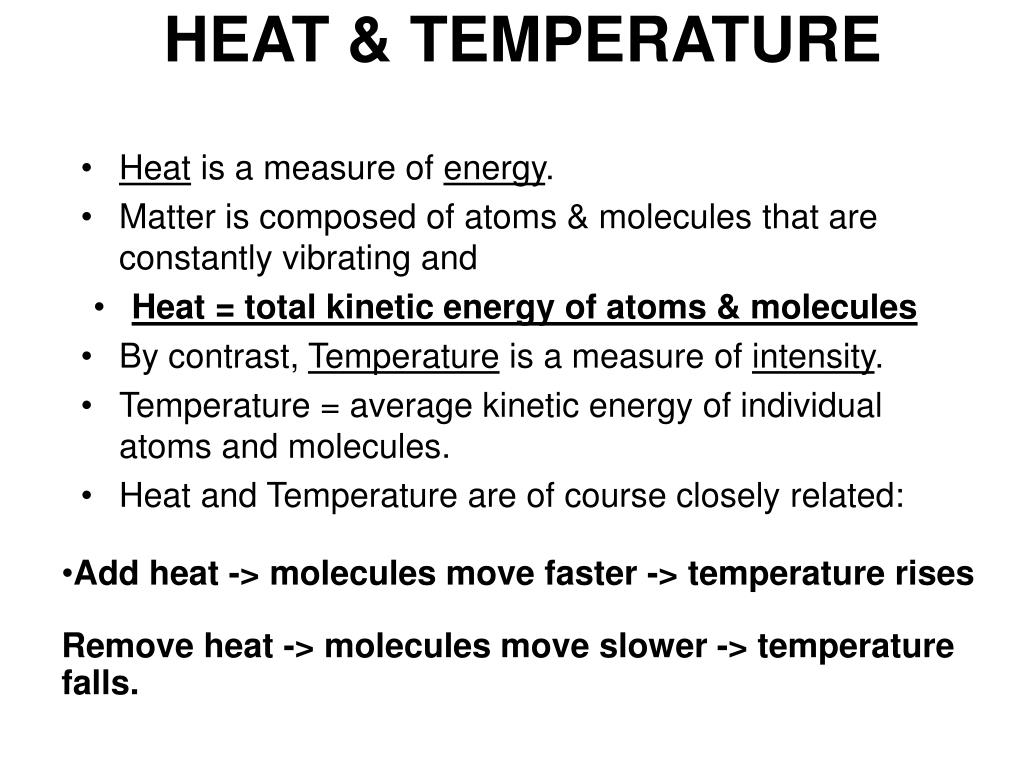
Heat Stroke vs. Heat Exhaustion: What's the Difference? - Healthline A temperature above 100°F (38°C) may indicate heat exhaustion while a temperature above 104°F (40°C) is a sign of heat stroke. Seek medical attention immediately if you believe you're having ... Heat vs Temperature - Difference and Comparison | Diffen Heat (symbol: Q) is energy. It is the total amount of energy (both kinetic and potential) possessed by the molecules in a piece of matter. Heat is measured in Joules. Temperature (symbol: T) is not energy. It relates to the average (kinetic) energy of microscopic motions of a single particle in the system per degree of freedom. Difference Between Heat and Temperature - Comparison & Measurement - BYJUS Heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of the molecules in the substance. The heat is dependent on factors like the speed of the particles, the size of the particles and the number of particles, etc. Heat and temperature (article) | Khan Academy Heat and temperature are two different but closely related concepts. Note that they have different units: temperature typically has units of degrees Celsius ( ^\circ\text C ∘C) or Kelvin ( \text K K ), and heat has units of energy, Joules ( \text J J ). Temperature is a measure of the average kinetic energy of the atoms or molecules in the system.
What is the Difference between Heat and Temperature? | Linquip There is a fine line that separates heat from temperature, in the sensation that heat is considered a form of energy, though temperature is a measure of energy. The difference between heat and temperature is small but significant; heat is the molecular motion's overall energy, while the temperature is the average energy of the molecular motion.
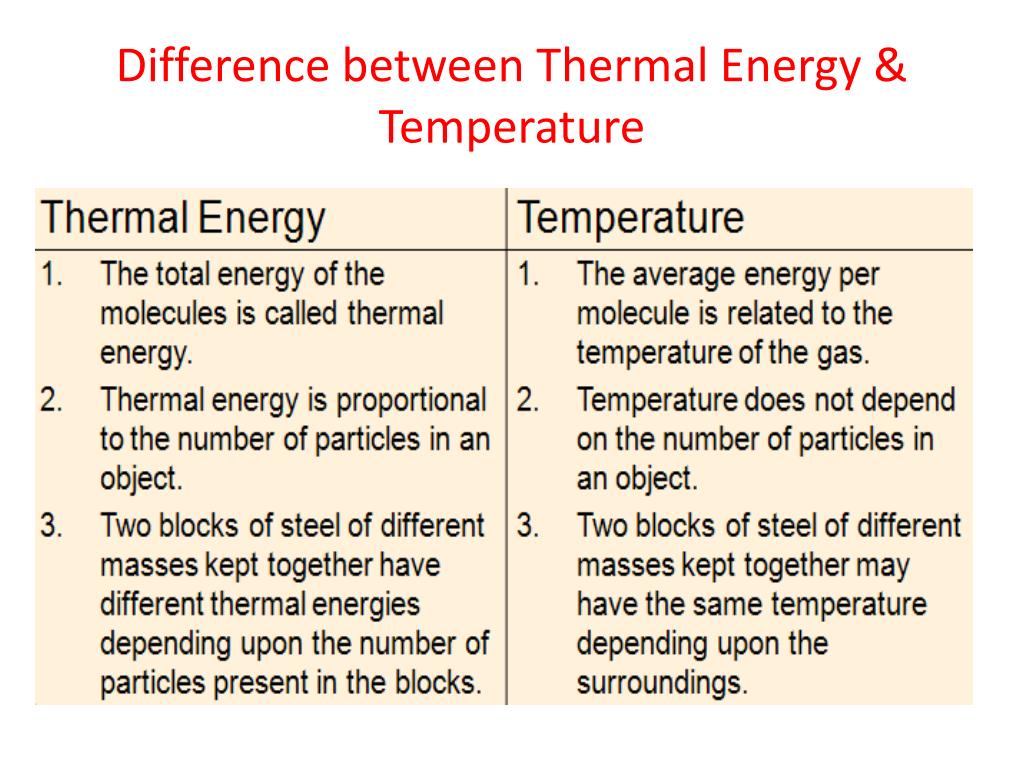
Difference Between Heat and Temperature (with Comparison Chart) - Key ... Heat measures both kinetic and potential energy contained by molecules in an object. On the other hand, temperature measures average kinetic energy of molecules in substance. The main feature of heat is that it travels from hotter region to cooler region. Unlike temperature, which rises when heated and falls when cooled.
Chemistry Flashcards | Quizlet Compare and contrast heat and temperature. Heat is a form of energy and temperature is measurement of the energy that a material is releasing. In which direction does energy flow when you touch an ice cube? From your finger to the ice because heat flows from warm to cold, not cold to warm.
2.2: Energy, Heat, and Temperature - Chemistry LibreTexts As a body loses or gains heat, its temperature changes in direct proportion to the amount of thermal energy q transferred: (2.2.3) q = C Δ T. The proportionality constant C is known as the heat capacity. (2.2.4) C = q Δ T. If Δ T is expressed in kelvins (degrees) and q in joules, the units of C are J K -1.
Heat vs. Temperature Comparison & Contrast | What is the Difference ... Though in casual usage they are often confused, heat and temperature are not the same. Heat measures the total energy, both kinetic and potential of the particles that make up some body....










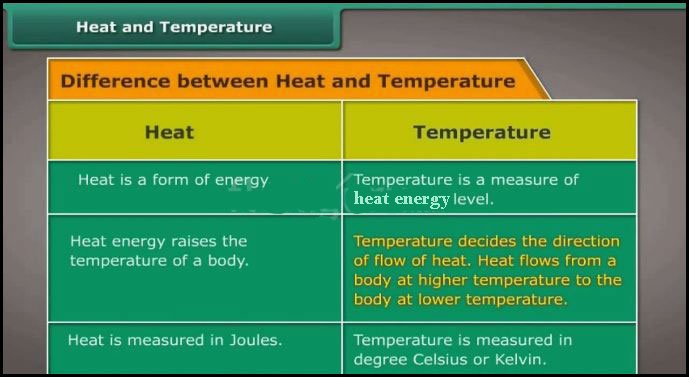











:max_bytes(150000):strip_icc()/metals-versusnonmetals-608809-v3-5b56348946e0fb0037001987.png)







0 Response to "40 compare and contrast heat and temperature"
Post a Comment