42 why does salt water not freeze
Does salt water ice stay frozen longer? - savbo.iliensale.com No, salt does not make ice longer. Salt causes ice to melt at a lower temperature than 0ºC (32ºF) this is why it's used to melt ice on roads. However, as salt. Trending; ... Freezing salt water for the cooler is the most important step to make ice last longer. Putting salt-water ice cubes in the cooler is a must-do. . Freezing Point of Salt Water - Science Struck Why Does the Salt Water Have Lower Freezing Point? Water molecules tend to form crystals in the process of freezing. In case of salt water, the process of crystallization is hampered by sodium (Na+) and chlorine (Cl-) ions present in it, and this, in turn, delays the process of freezing.
Why Does Honey Crystallize? | Heat Honey Safely With … May 18, 2021 · Make sure your honey is in a glass jar or jars (not plastic). Fill a pot with water that comes to ½ to ⅔ up the sides of the jars. Place jars of honey (sans lids) in a pot of water and bring to a gentle boil. Gently stir honey every few minutes to help break up crystals. Be careful not to splash any hot or boiling water into a jar of honey.

Why does salt water not freeze
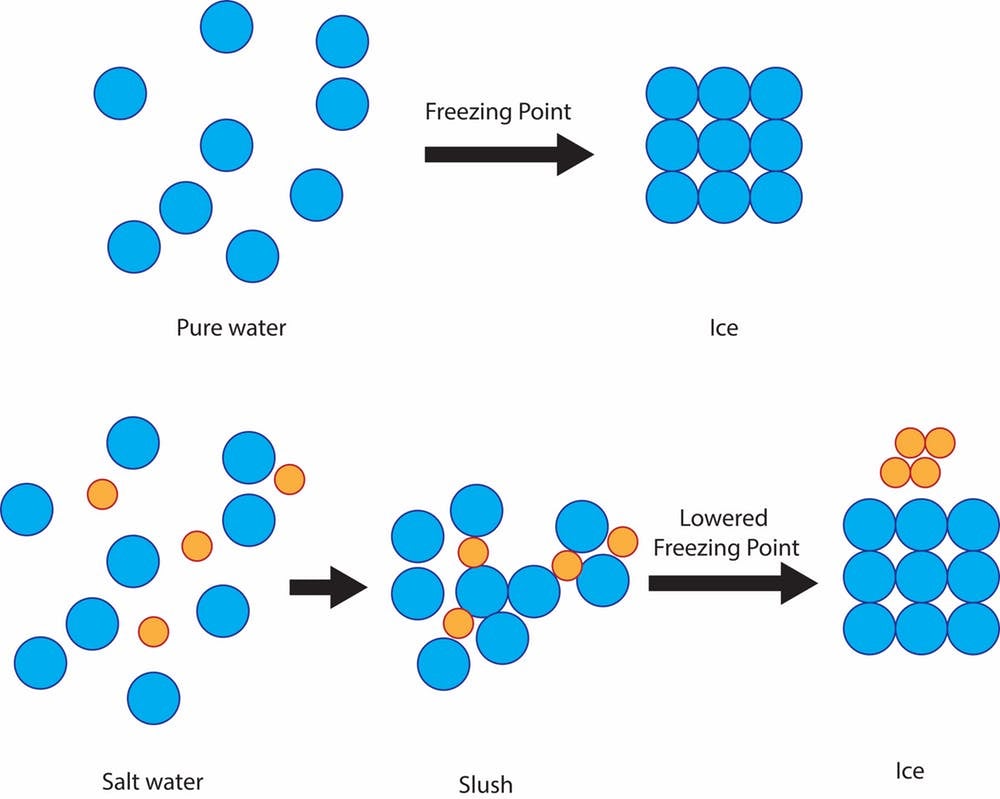
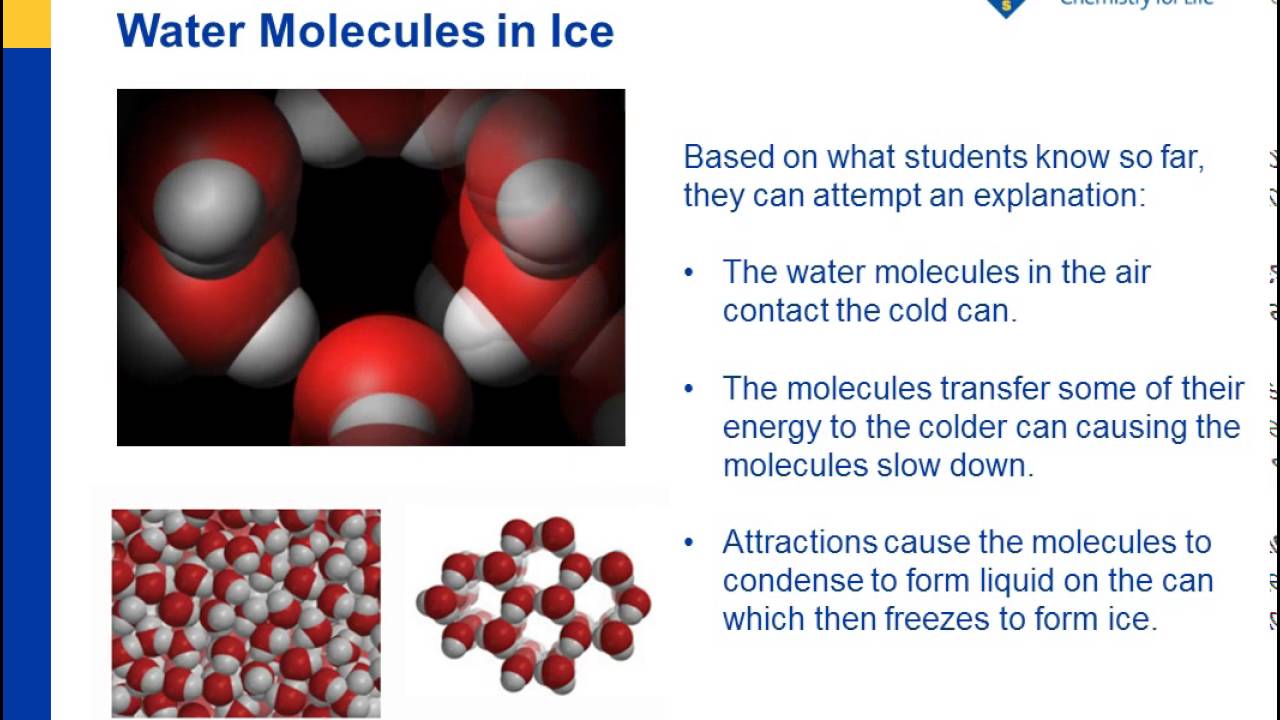
4 Ways to Make Clear Ice - wikiHow Dec 05, 2020 · Be careful not to put too little water in the bowl or the freezing will release enough heat to warm up the salt water to 0°C before the ice cubes finish freezing. The colder the freezer is, the higher the concentration of salt needs to be to prevent the salt water from freezing. Why doesn't seawater freeze at zero degrees? | Britannica The temperature drops, but look at this, the water doesn't freeze. The reason for this is tied to the sodium chloride ions in the salt water solution, shown here as blue and red circles. These charged particles disrupt the balance of the molecules, causing the number of water molecules that can hook onto ice molecules to decrease. Why does sea water does not freeze at 0°C? - Quora Because presence of salt causes depression of it's freezing point. As ice crystals are occupied by salt crystals first while freezing and thus for ice crystals to form more cooling is required. That's why freezing point of sea water is ~1.7 degree Celsius and not zero just like normal water lacking salt. Jumanah Idris
Why does salt water not freeze. Does Salt Water or Sugar Water Freeze Faster? - Reference.com Sugar water freezes faster than salt water, because salt has more molecules than sugar. Normally, water freezes at 32°F, however, when a substance is added to the water, it lowers its freezing point. This is not because of the chemical nature of the substance being added, but rather the number of molecules. This is referred to as a colligative property. What is reverse creaming, and why does it make great cake? 9.3.2022 · Perhaps she helped coin the name, as she described the method as “the reverse of starting by beating or creaming the butter.” (Rose, for what’s it’s worth, told me she’s not a fan of the “reverse creaming” name, but acknowledges that it’s what most people today call it.) Q & A: Freezing Saltwater - University of Illinois Urbana-Champaign Salt water will only freeze if it gets cold enough. For water as salty as it can get, that's -21°C. When you put salt on ice it will melt some of the ice but only if the temperature is above -21°C. So at any temperature where fully salty water will freeze, salt won't melt any ice. Ask Science - Quick and Dirty Tips “When I started following this podcast it was hard to understand all that Dr. Stierwalt says because I did not know about science. Nowadays, this is a must-subscribe podcast if you are a fan about learning how things work.” “This is a fun quick podcast to learn something interesting!”
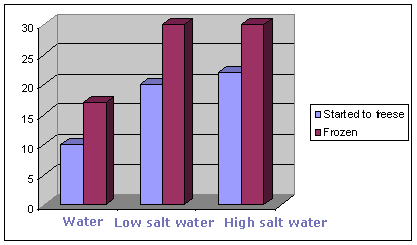
How Salt Melts Ice and Prevents Water From Freezing - ThoughtCo 5.5.2019 · Pure water freezes at 32°F (0°C). Water with salt (or any other substance in it) will freeze at some lower temperature. Just how low this temperature will be depends on the de-icing agent.If you put salt on ice in a situation where the temperature will never get up to the new freezing point of the salt-water solution, you won't see any benefit. Why Doesn't the Ocean Freeze? | Science project - Education This is why the ocean doesn't freeze: There's too much salt in it. Bodies of water located farther inland like islands and rivers have less salt in them, allowing them to freeze when the temperature drops to 0 degrees Celsius. Digging Deeper The beauty of science is that we never run out of opportunities to learn. How Long Does It Take for Saltwater to Freeze? - Reference.com Sea water freezes at a temperature between 28 and 29 degrees Fahrenheit. The freezing point for sea water is lower than it is for fresh water-which freezes at 32 degrees Fahrenheit-because of its greater density. 500 ml of saltwater (containing 1 tsp of salt) will take approximately four hours to freeze in a home freezer. What Temp Does Salt Water Freeze? | Jacks Of Science The freezing point of salt water is affected by a number of factors, including temperature. As the temperature decreases, the freezing point of salt water also decreases. In addition, the presence of impurities can also affect the freezing point of salt water. For example, impurities such as clay or sand can lower the freezing point of salt water.
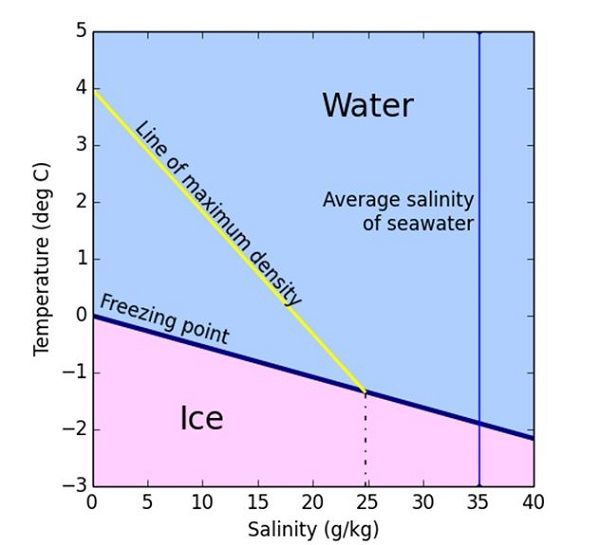
Freezing Point of Saltwater | Physics Van | UIUC As the water starts to freeze, the salt gets left in the liquid. So if you start out with water that isn't saturated with salt, as it freezes the leftover water will get saturated. So if the water starts to freeze at, for example, -10°C, more will freeze as it's cooled further until finally the last bit will freeze at -21.1°C. Why Does Salt Melt Ice? Understanding How It Works - ThoughtCo Salt melts ice and help prevent re-freezing by lowering the freezing point of water. This phenomenon is called freezing point depression. The working temperature range isn't the same for all types of salt. For example, calcium chloride lowers the freezing point more than sodium chloride. In addition to melting ice, freezing point depression can ... Why Does Salt Lower the Melting Point of Ice - WorldOfChemicals As a result of these salts, seawater is denser than both freshwater and pure water. Therefore the freezing point of seawater decreases as salt concentration increases. Although the saltiness of ocean water varies, this lowers the freezing point of ocean water to about -1.8°C or 28.8°F. So ocean water will freeze. MIT School of Engineering | » Does hot water freeze faster than … But logic triumphs when it comes the plain ordinary water that comes from the household faucet. Most likely to impact the freezing point of water is the presence of impurities such as salt, dissolved solids and gases — and the ingredients of homemade ice cream. Thanks to Khubaib Mukhtar of Pakistan for this question. April 30, 2013
Why does salty water take longer to freeze? - Quora Ocean water freezes just like freshwater, but at lower temperatures. Fresh water freezes at 32 degrees Fahrenheit but seawater freezes at about 28.4 degrees ...
Why does salt water not freeze? - Answers Why doesn't saltwater freeze? Salt water will freeze but at a lower temperature than fresh water. The point at which salt water freezes depends on the amount of salt in the solution. Which water...
Can salt water be desalinated by freezing? - phe.motoretta.ca Fresh water, on the other hand, is most dense while still at 39.2 degrees Fahrenheit , well above the freezing point. Why does salt water not freeze? The reason for this is tied to the sodium chloride ions in the salt water solution, shown here as blue and red circles. These charged particles disrupt the balance of the molecules, causing the ...
Fresh and Salt Water Experiment | Ask A Biologist In your "salt water" container, dissolve 1.5 teaspoons of salt for every cup of water (so, if you have two cups, use 3 teaspoons). Put the containers in a freezer (this will take at few hours to freeze, best done overnight) and keep frozen until ready to perform experiment Take the ice out of the containers and set them next to each other.
10 Differences Between Freeze Dried vs Dehydrated Food Mar 30, 2022 · Freeze-drying removes water only. Since it does not involve heating, the nutrients, flavors, and color of the food are left intact and remain so for quite a long time with proper storage. Worth noting, this is what makes most nutritionist and food experts to recommend freeze-drying when dealing with herbs.
Does Salt Water Freeze? | Wonderopolis The freezing point of freshwater is 0° Celsius or 32° Fahrenheit. The presence of salt in water, though, reduces the freezing point of water. The more salt in ...
Does the salt water in Earth's oceans freeze? If so, why or why not? Ocean water freezes just like freshwater, but at lower temperatures. Fresh water freezes at 32 degrees Fahrenheit but seawater freezes at about 28.4 degrees Fahrenheit, because of the salt in it. When seawater freezes, however, the ice contains very little salt because only the water part freezes. It can be melted down to use as drinking water.
Do salt water freeze? Explained by FAQ Blog Does adding salt to water lower the freezing point? When added to ice, salt first dissolves in the film of liquid water that is always present on the surface, thereby lowering its freezing point below the ices temperature.Ice in contact with salty water therefore melts, creating more liquid water, which dissolves more salt, thereby causing more ice to melt, and so on.
Which freezes faster, water or salt water? - UC Santa Barbara Answer 1: While pure water freezes at 0°C (32°F), salt water needs to be colder before it freezes and so it usually takes longer to freeze. The more salt in the water, the lower the freezing point. Very salty water freezes at around -21 °C, or about -6 °F. What is interesting is that this effect is used all over the place for practical reasons.
Why don't the oceans freeze? - Science Questions with Surprising Answers 1. Salt The high concentration of salt in ocean water lowers its freezing point from 32° F (0° C) to 28° F (-2° C). As a result, the ambient temperature must reach a lower point in order to freeze the ocean than to freeze freshwater lakes. This freezing-point depression effect is the same reason we throw salt on icy sidewalks in the winter.
UCSB Science Line The salt content in the water lowers its freezing temperature by 32°F (17.8°C)! This is a general phenomenon too - dissolving a small amount of any solid in any liquid will decrease its freezing point and increase its boiling point, regardless of what the solid and liquid are. These effects are called the colligative properties of solutions.
Scientific Method Scenarios - The Biology Corner Ask the group to design and experiment to answer the experimental question. Students should identify a control group, dependent and independent variables and possible outcomes or what type of data would be gathered. Stress to students that they will not actually be …
Does Salt Water Freeze? | Wonderopolis The presence of salt in water, though, reduces the freezing point of water. The more salt in the water, the lower the freezing point will be. When freshwater freezes, water molecules of hydrogen and oxygen have bonded together into a crystalline structure of ice.
Can Sea Water Freeze? - NASA Aquarius Mission Salt is excluded in the formation of ice; therefore ice made from salt water is essentially salt-free.7 pages
Does salt help freeze water? Explained by FAQ Blog Why does salt water not freeze? The reason for this is tied to the sodium chloride ions in the salt water solution, shown here as blue and red circles. These charged particles disrupt the balance of the molecules, causing the number of water molecules that can hook onto ice molecules to decrease. Water thus freezes at a slower rate.
Can salt water freeze? Explained by FAQ Blog When added to ice, salt first dissolves in the film of liquid water that is always present on the surface, thereby lowering its freezing point below the ices temperature. Ice in contact with salty water therefore melts, creating more liquid water, which dissolves more salt, thereby causing more ice to melt, and so on. Does salt water melt slower?
INVESTIGATION: Dasani Water Does Not Freeze Like Normal Water Another reason why water would not turn into ice even when being placed into a freezer is that it is heavily filtered. In this case, all the impurities are removed from the liquid. Finally, water may not solidify at all because of certain chemicals that were added to the bottled water and prevent freezing, for instance, propylene glycol.
Can the ocean freeze? - National Ocean Service Ocean water freezes just like freshwater, but at lower temperatures. Fresh water freezes at 32 degrees Fahrenheit but seawater freezes at about 28.4 degrees Fahrenheit, because of the salt in it. When seawater freezes, however, the ice contains very little salt because only the water part freezes. It can be melted down to use as drinking water.
Salt and Fresh Water Experiment May 30, 2019 · Specific amounts of salt water is heavier than the same volume of freshwater. When salt is dissolved in water, like at the ocean, the salt adds to the mass of the water. The salt makes the water denser than it would be without the salt. When salt is dissolved in water, as it is in ocean water, it adds to the mass of the water and makes the ...
Does freshwater freeze faster than saltwater? Why does salt water freeze slower? The reason for this is tied to the sodium chloride ions in the salt water solution, shown here as blue and red circles. These charged particles disrupt the balance of the molecules, causing the number of water molecules that can hook onto ice molecules to decrease. Water thus freezes at a slower rate.
Why Doesn't the Ocean Freeze? | Mental Floss Salt has small particles called ions that surround the water molecules and keep them from sticking together to form ice. Ice will only begin to form when the ocean water gets even colder—about...
Why does sea water does not freeze at 0°C? - Quora Because presence of salt causes depression of it's freezing point. As ice crystals are occupied by salt crystals first while freezing and thus for ice crystals to form more cooling is required. That's why freezing point of sea water is ~1.7 degree Celsius and not zero just like normal water lacking salt. Jumanah Idris
Why doesn't seawater freeze at zero degrees? | Britannica The temperature drops, but look at this, the water doesn't freeze. The reason for this is tied to the sodium chloride ions in the salt water solution, shown here as blue and red circles. These charged particles disrupt the balance of the molecules, causing the number of water molecules that can hook onto ice molecules to decrease.
4 Ways to Make Clear Ice - wikiHow Dec 05, 2020 · Be careful not to put too little water in the bowl or the freezing will release enough heat to warm up the salt water to 0°C before the ice cubes finish freezing. The colder the freezer is, the higher the concentration of salt needs to be to prevent the salt water from freezing.



























0 Response to "42 why does salt water not freeze"
Post a Comment