42 chemical reactions that release energy
Biochemistry - Wikipedia WebBiochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and metabolism.Over the last decades of the 20th century, biochemistry has become successful at explaining living … Chemical reactions and energy Flashcards | Quizlet Exothermic A (n) ________ reaction is a chemical reaction that releases energy. bonds Energy released during a chemical reaction comes from chemical _____ between atoms. more In an exothermic reaction, the bonds in the reactants contain ____ energy than the bonds in the products. thermal
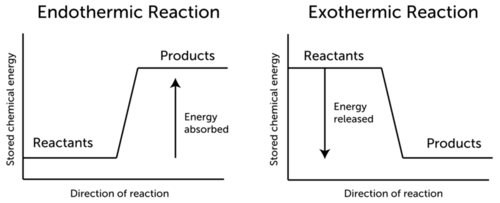
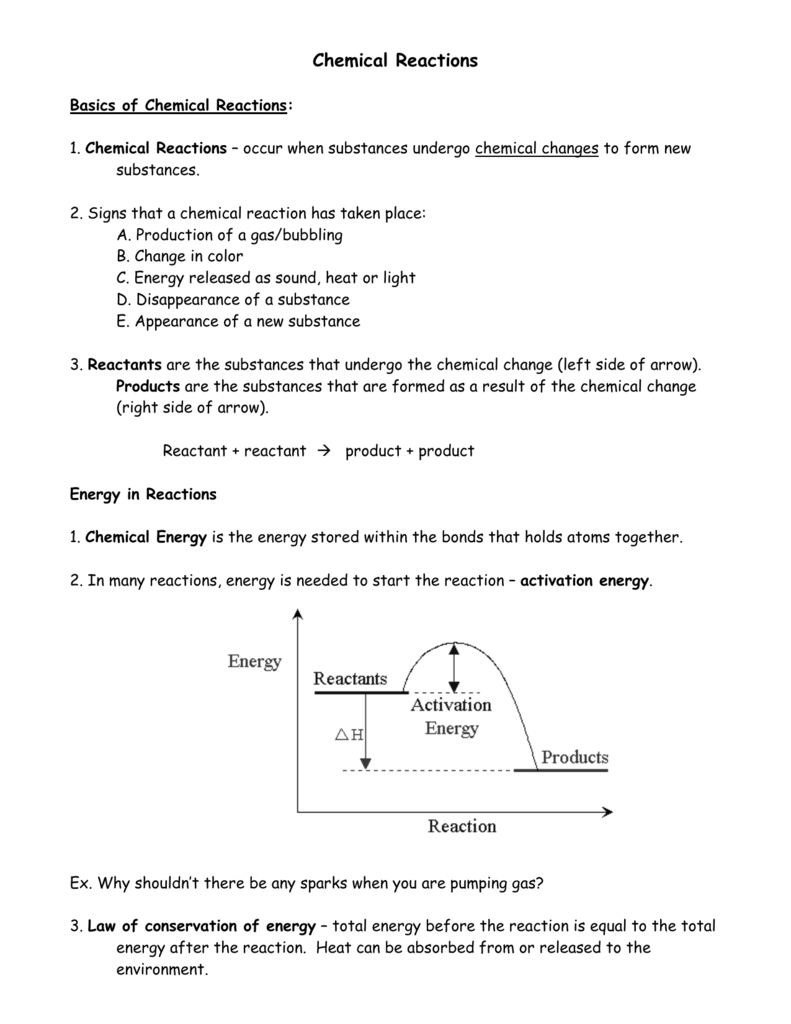
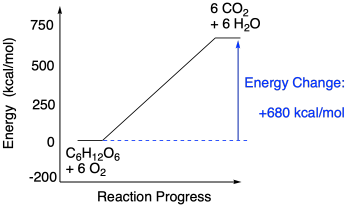
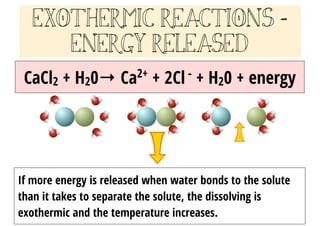
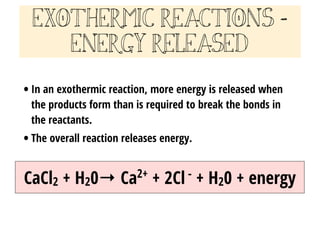
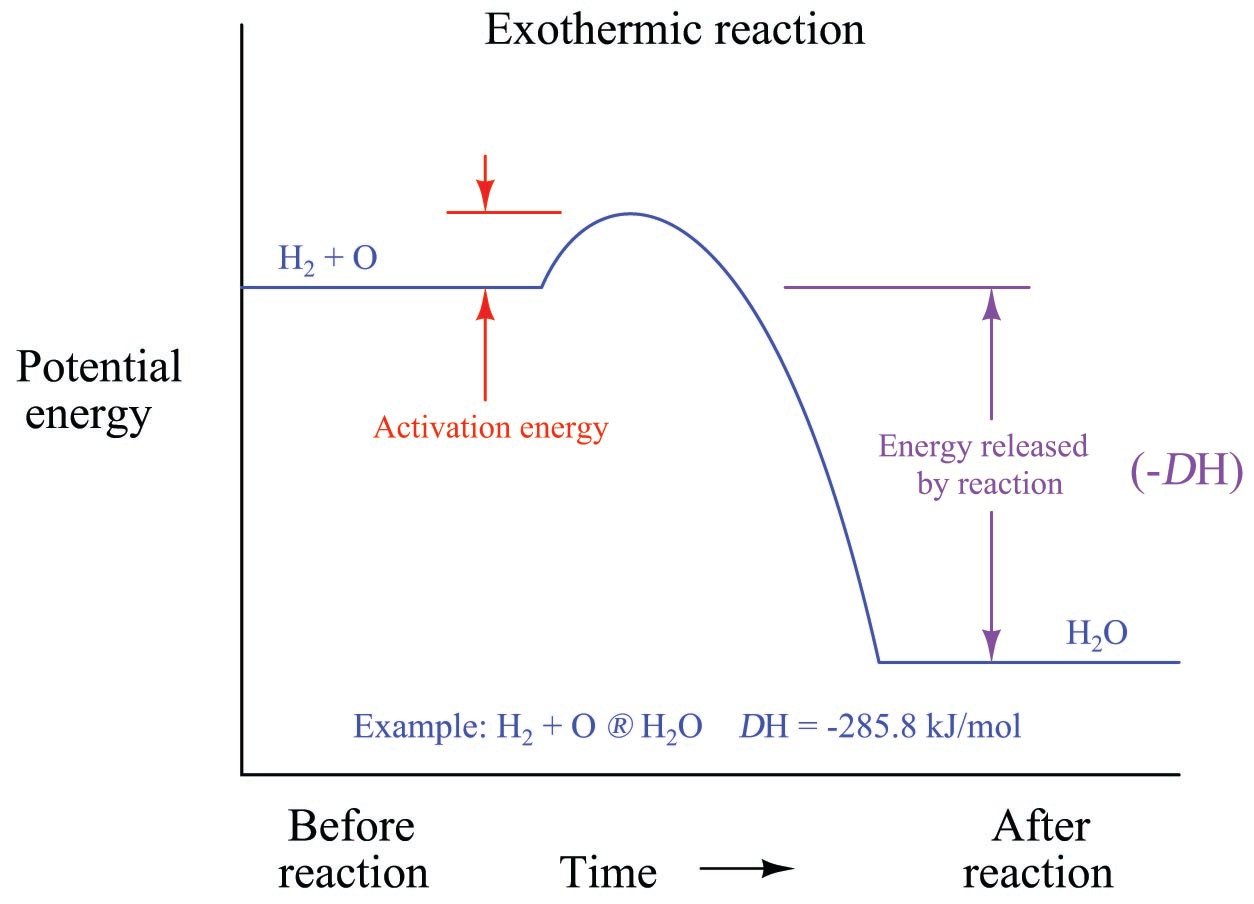
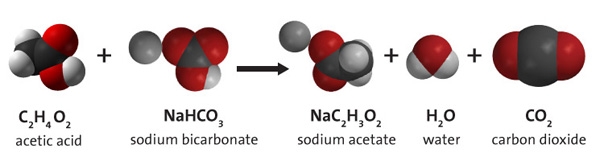
3.9 Energy in Chemical Reactions - Human Biology A chemical reaction that releases energy is called an exothermic reaction . This type of reaction can be represented with this general chemical equation: Reactants → Products + Heat Another example of an exothermic reaction is chlorine combining with sodium to form table salt.
Chemical reactions that release energy
Exothermic Reactions Endothermic Reactions energy changes … Web1(c) Heat Changes in Chemical Reactions - reaction profile diagrams When chemical reactions occur, as well as the formation of the products - the chemical change, there is also a heat energy change which can often be detected as a temperature change.; This means the products have a different energy content than the original reactants (see the … Energy in Chemical Reactions | exothermic & endothermic Burning is an exothermic chemical reaction in which a substance combines with oxygen to produce heat along with light, carbon dioxide, and water. Rapid Release Sometimes the energy is released rapidly. For example, charcoal lighter fluid combines with oxygen in the air and produces enough heat to ignite a charcoal fire within a few minutes. Chemical Reactions and Equations Class 10 Notes CBSE Science WebChemical Change: A change that results in the formation of one or more new compounds. Chemical changes are also known as chemical reactions. Observations in a Chemical Reaction. In a chemical reaction or chemical change, the following observations can be made, Formation of new substances. Change in mass. Changes in energy. Evolution of …
Chemical reactions that release energy. How Does Energy Change During A Chemical Reaction Chemical reactions that absorb energy require a source of energy. The energy needed to get a reaction started is called the activation energy. Enzymes An enzyme is a protein that acts as biological catalyst. A catalyst is a substance that speeds up the rate of a chemical reaction. How is energy released during bond formation? Is Energy Released When Chemical Bonds Are Formed? Web17/07/2019 · Exothermic reactions release energy in the form of heat, so the sum of the energy released exceeds the amount required. Endothermic reactions absorb energy, so the sum of the energy required exceeds the amount that is released. In all types of chemical reactions, bonds are broken and reassembled to form new products. … Chemical thermodynamics - Wikipedia WebChemical energy is the energy that can be released when chemical substances undergo a transformation through a chemical reaction.Breaking and making chemical bonds involves energy release or uptake, often as heat that may be either absorbed by or evolved from the chemical system.. Energy released (or absorbed) because of a reaction between … 12 Examples of Chemical Energy - ThoughtCo Web25/09/2019 · Petroleum: Can be burned to release light and heat or changed into another form of chemical energy, such as gasoline. Chemical batteries: Store chemical energy to be changed into electricity. Biomass: Combustion reaction converts chemical energy into light and heat. Natural gas: Combustion reaction converts chemical energy into light and …
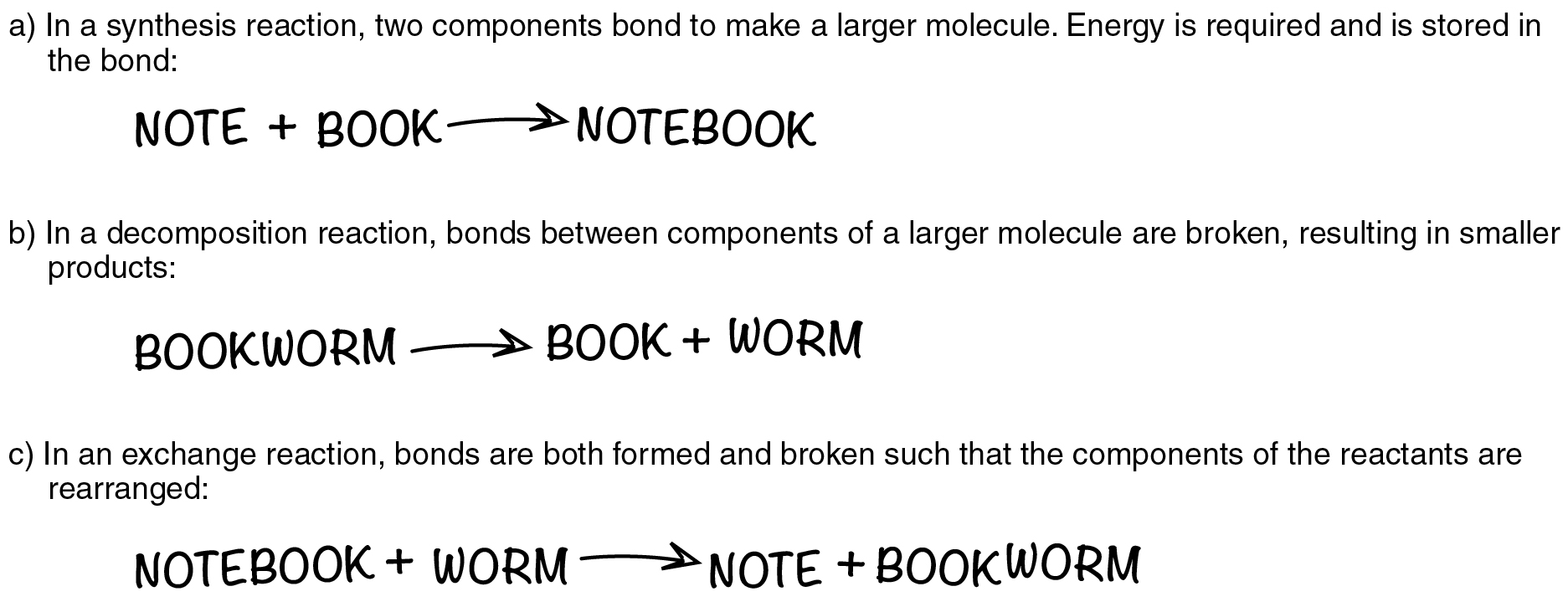
5 Dimension 3: Disciplinary Core Ideas - Physical Sciences | A ... WebSome chemical reactions release energy, others store energy. By the end of grade 12. Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in total binding energy (i.e., the sum of all bond … What is the energy released in chemical reactions? Often, the energy we experience in chemical reactions can be adequately described by the transfer of heat, and therefore thermochemistry and thermodynamics take up a large portion of our instruction in Chemistry. Other types of energy, such as electromagnetic radiation, electrochemical, and nuclear, can all be described in terms of potential ... equationbalancer.com › blog › chemical-reaction-and5 types of Chemical Reactions with Chemical Reaction examples Jun 24, 2021 · And if a person learns about these basic chemical reactions, dealing with chemical equations would not be a problem. The list of chemical reactions in chemistry is as follows. Combination reaction; Decomposition reaction; Combustion reaction; Single replacement reaction; Double replacement reaction Why Do Nuclear Reactions Release More Energy Than Chemical The energy released in a chemical reaction such as hydrogen and oxygen combining to produce a water molecule is about 1 eV per reaction so fusion fuel releases more than a million times as much energy per gram as typical chemical fuels. Why Does nuclear fission create energy? In nuclear fission atoms are split apart which releases energy.
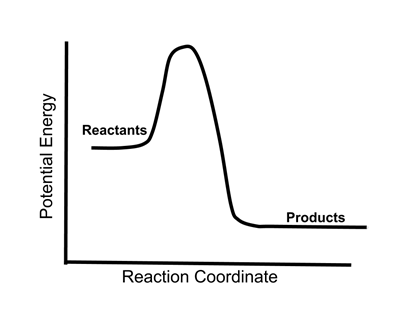
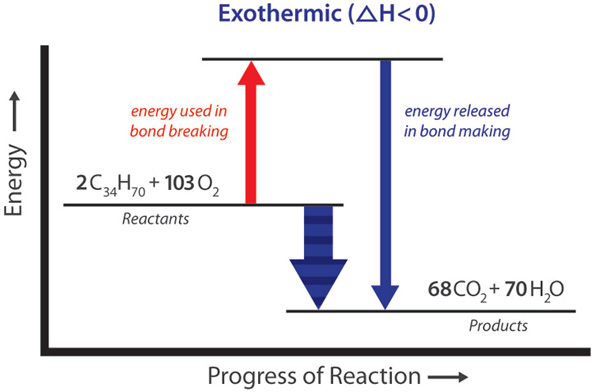
chemical reaction - Energy considerations | Britannica Energy plays a key role in chemical processes. According to the modern view of chemical reactions, bonds between atoms in the reactants must be broken, and the atoms or pieces of molecules are reassembled into products by forming new bonds. Energy is absorbed to break bonds, and energy is evolved as bonds are made. In some reactions the energy required to break bonds is larger than the energy ... Energy Changes in Chemical Reactions - Course Hero Excess energy from the reaction is released as heat and light. Chemical reaction A thermite reaction, which produces molten iron. Endothermic Reactions Endothermic reactions, on the other hand, absorb heat and/or light from their surroundings. For example, decomposition reactions are usually endothermic. nap.nationalacademies.org › read › 131655 Dimension 3: Disciplinary Core Ideas - Physical Sciences ... Chemical reactions, which underlie so many observed phenomena in living and nonliving systems alike, conserve the number of atoms of each type but change their arrangement into molecules. Nuclear reactions involve changes in the types of atomic nuclei present and are key to the energy release from the sun and the balance of isotopes in matter. 5 types of Chemical Reactions with Chemical Reaction examples Web24/06/2021 · Also find how to write a net ionic equation and how redox reaction balancer calculator helps you to balance redox equations.. What are the types of Chemical Reactions in Chemistry? There are 5 types of chemical reactions in chemistry which are important. Among different types of reaction in chemistry, these 5 types of chemical …
Chemical Reactions for 7th Grade Chemistry - MS-PS1-1, MS ... - OpenSciEd WebTake your 7th grade chemistry lessons to the next level! Our chemical reactions unit for middle school science will engage your students while meeting MS-PS1-1, MS-PS1-2, MS-PS1-5, and MS-LS1-8.
Chemical Reactions That Release Energy - Quizlet Chemical Reactions That Release Energy Discover free flashcards, games, and test prep activities designed to help you learn about Chemical Reactions That Release Energy and other concepts. They're customizable and designed to help you study and learn more effectively. BROWSE SIMILAR CONCEPTS Second Law Of Thermodynamics First Law Of Thermodynamics
› when-energy-is-released-inIs Energy Released When Chemical Bonds Are Formed? - ThoughtCo Jul 17, 2019 · Exothermic reactions release energy in the form of heat, so the sum of the energy released exceeds the amount required. Endothermic reactions absorb energy, so the sum of the energy required exceeds the amount that is released. In all types of chemical reactions, bonds are broken and reassembled to form new products.
Chemical Reactions and Energy - CliffsNotes The oxidation-reduction reactions performed by the coenzymes and other molecules are essential to the energy metabolism of the cell. Other molecules participating in this energy reaction are called cytochromes. Together with the enzymes, cytochromes accept and release electrons in a system referred to as the electron transport system.
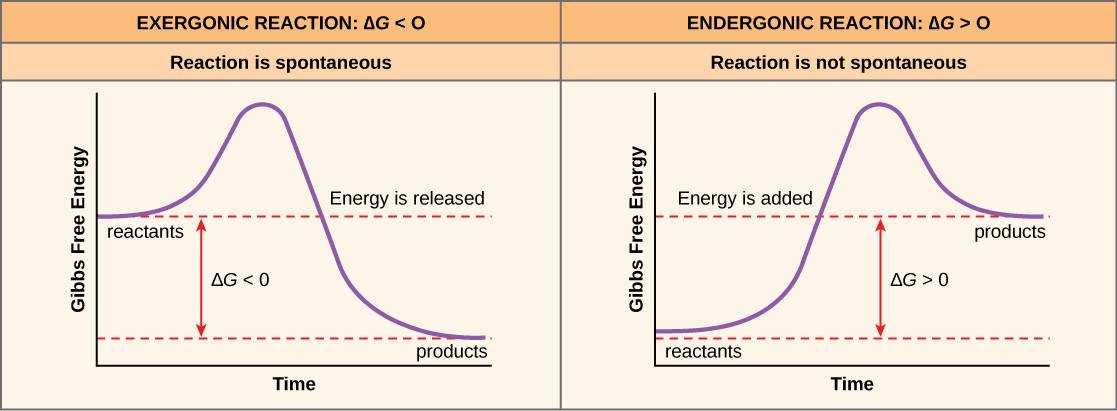
PDF Chemical Reactions and Energy - Pearland High School •Some chemical reactions release energy. •Exergonic •Digesting polymers. •Hydrolysis = catabolism. digesting molecules= LESS organization= lower energy state building molecules= MORE organization= higher energy state Endergonic vs. Exergonic Reactions Exergonic Endergonic- G + G G = change in free energy = ability to do work Free energy ...
PDF Energy from Chemical Reactions - Oak Ridge Institute for Science and ... Energy from Chemical Reactions . Submitted by: Tammy Guthrie, Secondary Science . Hellstern Middle School, Springdale, Arkansas ... test, and modify a device that either releases or absorbs thermal energy by chemical processes. MS-PS3-5 Construct, use, and present arguments to support the claim that when the kinetic energy of an object changes ...
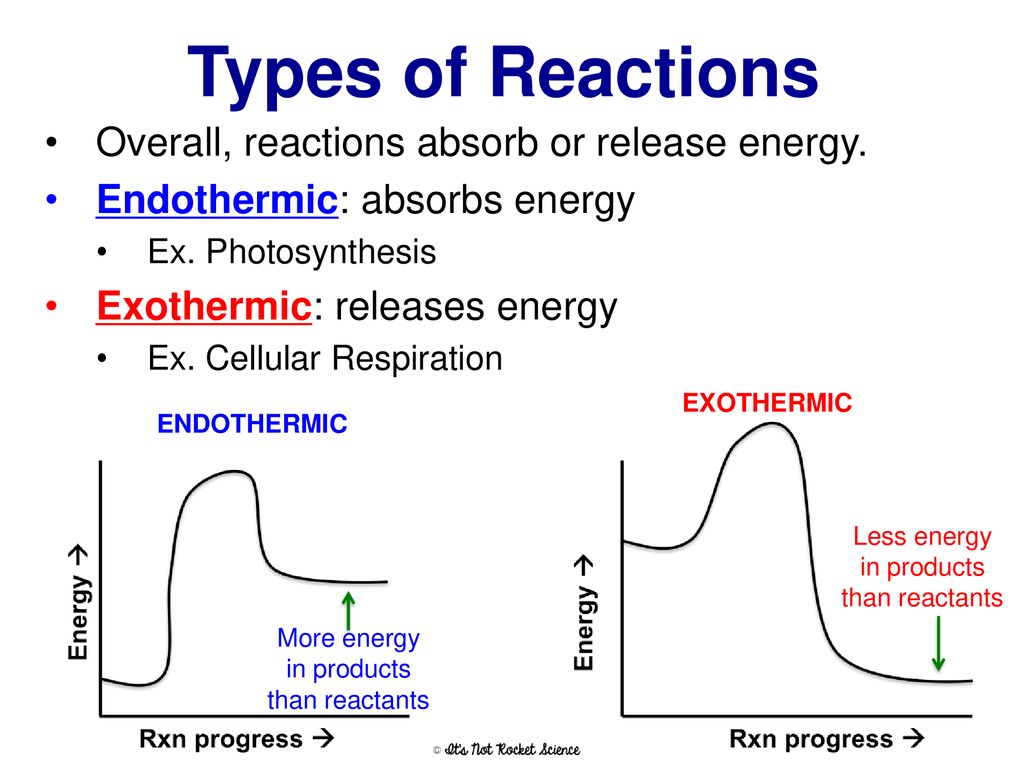
highschoolenergy.acs.org › content › hsefExothermic, Endothermic, & Chemical Change | Energy ... By comparing the energy used when bonds in the reactants are broken with the energy released when bonds in the products are formed, you can determine whether a chemical reaction releases energy or absorbs energy overall. Chemical reactions that release energy are called exothermic. In exothermic reactions, more energy is released when the bonds ...
How Cells Obtain Energy from Food - Molecular Biology of the Cell ... The energy that the electrons release in this process is used to pump H + ions (protons) across the membrane—from the inner mitochondrial compartment to the outside (Figure 2-81). A gradient of H + ions is thereby generated. This gradient serves as a source of energy, being tapped like a battery to drive a variety of energy-requiring reactions.
en.wikipedia.org › wiki › Chemical_thermodynamicsChemical thermodynamics - Wikipedia Chemical energy is the energy that can be released when chemical substances undergo a transformation through a chemical reaction.Breaking and making chemical bonds involves energy release or uptake, often as heat that may be either absorbed by or evolved from the chemical system.
› revision-notes › cbse-class-10Chemical Reactions and Equations Class 10 Notes ... - VEDANTU Chemical Change: A change that results in the formation of one or more new compounds. Chemical changes are also known as chemical reactions. Observations in a Chemical Reaction. In a chemical reaction or chemical change, the following observations can be made, Formation of new substances. Change in mass. Changes in energy. Evolution of gas.
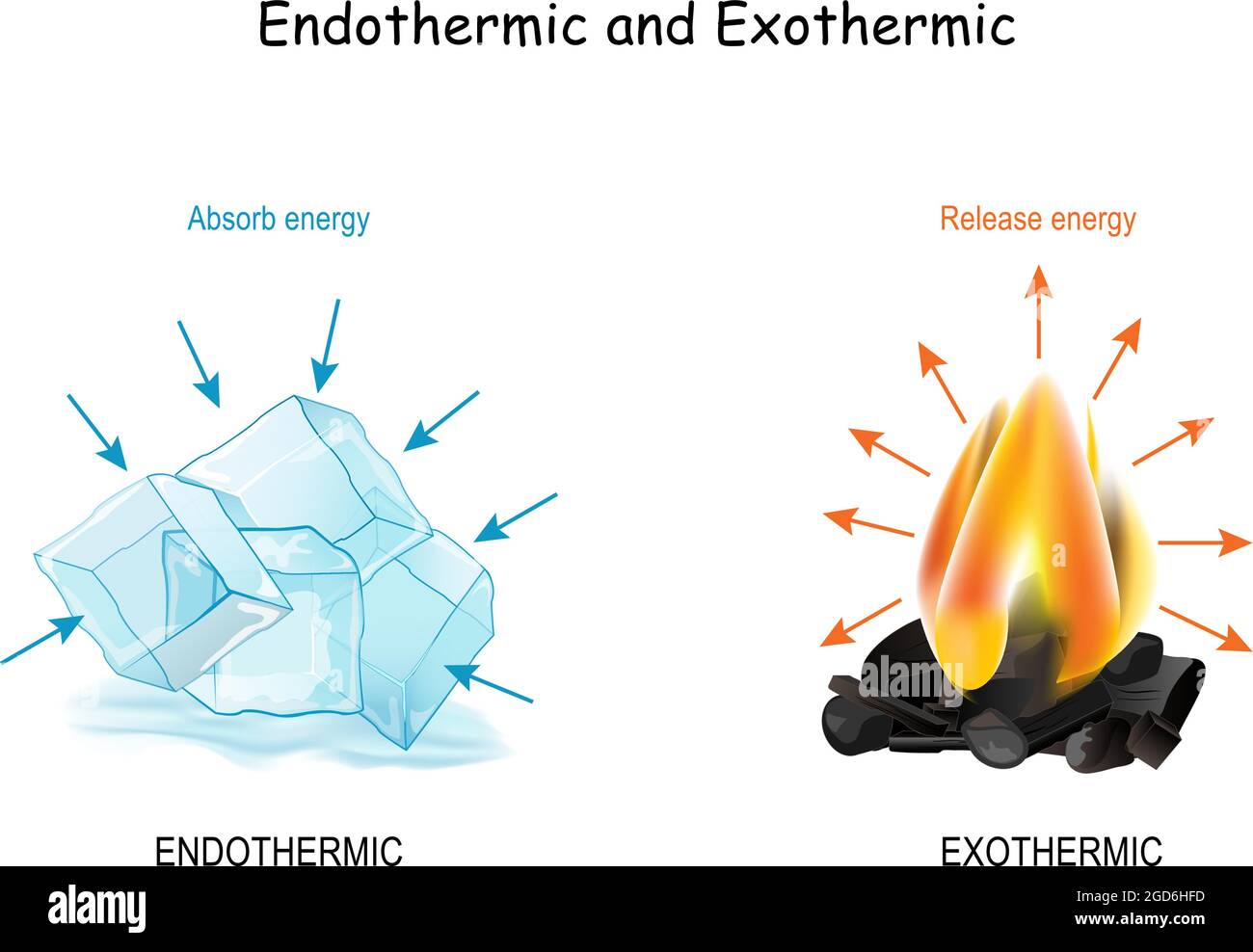
Why Do Some Chemical Reactions Release Heat While Others Absorb Heat These reactions called exothermic reactions release energy. In other chemical reactions it takes more energy to break bonds in reactants than is released when bonds form in products. These reactions called endothermic reactions absorb energy.Aug 16 2018 Why do some reactions release heat while others absorb heat?
What are Endothermic Reactions? (with Examples & Video) - BYJUS The energy supplied/released can be of various forms (such as heat, light, and electricity). Endothermic reactions generally involve the formation of chemical bonds through the absorption of heat from the surroundings. On the other hand, exothermic reactions involve the release of heat energy generated from bond-breakage.
Chemical energy - Energy Education Almost any molecule that's a collection of carbon atoms (especially with hydrogen and oxygen) will react with oxygen to form carbon dioxide. Wood and other materials can be burned when there is oxygen present in what is called a combustion reaction. Burning wood transforms this chemical potential energy and releases it as radiant heat.
What chemical reactions release energy? | Socratic What chemical reactions release energy? Chemistry Thermochemistry Energy Change in Reactions 1 Answer G_Ozdilek Aug 29, 2016 Exothermic reactions. Explanation: When heat is liberated after the reaction, it is called exothermic reaction. Answer link
› example-of-chemical-energy-60926012 Examples of Chemical Energy - ThoughtCo Sep 25, 2019 · Biomass: Combustion reaction converts chemical energy into light and heat. Natural gas: Combustion reaction converts chemical energy into light and heat. Food: Digested to convert chemical energy into other forms of energy used by cells. Cold packs: Chemical energy is absorbed in a reaction. Propane: Burned to produce heat and light.
Energy in Chemical Reactions | Chemistry in Industrial ... - control A chemical reaction resulting in a net release of energy is called exothermic. Conversely, a chemical reaction requiring a net input of energy to occur is called endothermic. The relationship between chemical reactions and energy exchange corresponds to the breaking or making of chemical bonds.
When reactions release energy? - buysch.adamstankandlift.com Chemical reactions that release energy are called exothermic. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds in the reactants. Exothermic reactions are accompanied by an increase in temperature of the reaction mixture. What happens when a reaction releases energy?
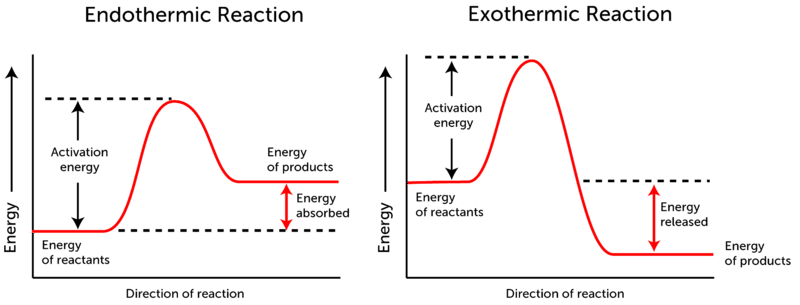
Why do some reactions release energy, while others absorb energy from ... Endothermic reaction As you can see, endothermic reaction is the opposite of an exothermic reaction. The reactants are at a lower energy level than the products. Therefore, in endothermic reactions, the enthalpy change is always greater than zero (∆H>0). And for the reaction to occur, it must absorb energy from the surroundings.
Chemical Energy - Knowledge Bank - Solar Schools What is chemical energy? Chemical energy is stored in the bonds that connect atoms with other atoms and molecules with other molecules. Because chemical energy is stored, it is a form of potential energy. When a chemical reaction takes place, the stored chemical energy is released.. Heat is often produced as a by-product of a chemical reaction - this is called an exothermic reaction.
Exothermic & Endothermic Reactions
What are Exothermic Reactions? (with Examples and Video) - BYJUS An Exothermic reaction is a chemical reaction that involves the release of energy in the form of heat or light. These reactions are the opposite of endothermic reactions and can be expressed in a chemical equation as follows: Reactants → Products + Energy.
Do reactions release energy? - goldsch.adamstankandlift.com What energy can be released by a chemical reaction? Chemical energy, Energy stored in the bonds of chemical compounds. Chemical energy may be released during a chemical reaction, often in the form of heat; such reactions are called exothermic.Reactions that require an input of heat to proceed may store some of that energy as chemical energy in newly formed bonds.
What Happens To Energy In A Chemical Reaction - Realonomics Chemical reactions that release energy are called exothermic. In exothermic reactions more energy is released when the bonds are formed in the products than is used to break the bonds in the reactants. Exothermic reactions are accompanied by an increase in temperature of the reaction mixture. Is energy conserved in a chemical reaction?
How Is Energy Involved In A Chemical Reaction - Realonomics How Is Energy Involved In A Chemical Reaction? All chemical reactions involve energy. Energy is used to break bonds in reactants and energy is released when new bonds form in products. Endothermic reactions absorb energy and exothermic reactions release energy. The law of conservation of energy states that matter cannot be created or destroyed.
Exothermic, Endothermic, & Chemical Change | Energy … WebBy comparing the energy used when bonds in the reactants are broken with the energy released when bonds in the products are formed, you can determine whether a chemical reaction releases energy or absorbs energy overall. Chemical reactions that release energy are called exothermic. In exothermic reactions, more energy is released when …
Chemical reactions that release energy? - Answers Chemical reaction that release energy? All chemical reactions that release energy in the form of heat are called exothermic reactions. Describe the role of energy in chemical reactions? Chemical...
Chemical Reactions and Equations Class 10 Notes CBSE Science WebChemical Change: A change that results in the formation of one or more new compounds. Chemical changes are also known as chemical reactions. Observations in a Chemical Reaction. In a chemical reaction or chemical change, the following observations can be made, Formation of new substances. Change in mass. Changes in energy. Evolution of …
Energy in Chemical Reactions | exothermic & endothermic Burning is an exothermic chemical reaction in which a substance combines with oxygen to produce heat along with light, carbon dioxide, and water. Rapid Release Sometimes the energy is released rapidly. For example, charcoal lighter fluid combines with oxygen in the air and produces enough heat to ignite a charcoal fire within a few minutes.
Exothermic Reactions Endothermic Reactions energy changes … Web1(c) Heat Changes in Chemical Reactions - reaction profile diagrams When chemical reactions occur, as well as the formation of the products - the chemical change, there is also a heat energy change which can often be detected as a temperature change.; This means the products have a different energy content than the original reactants (see the …













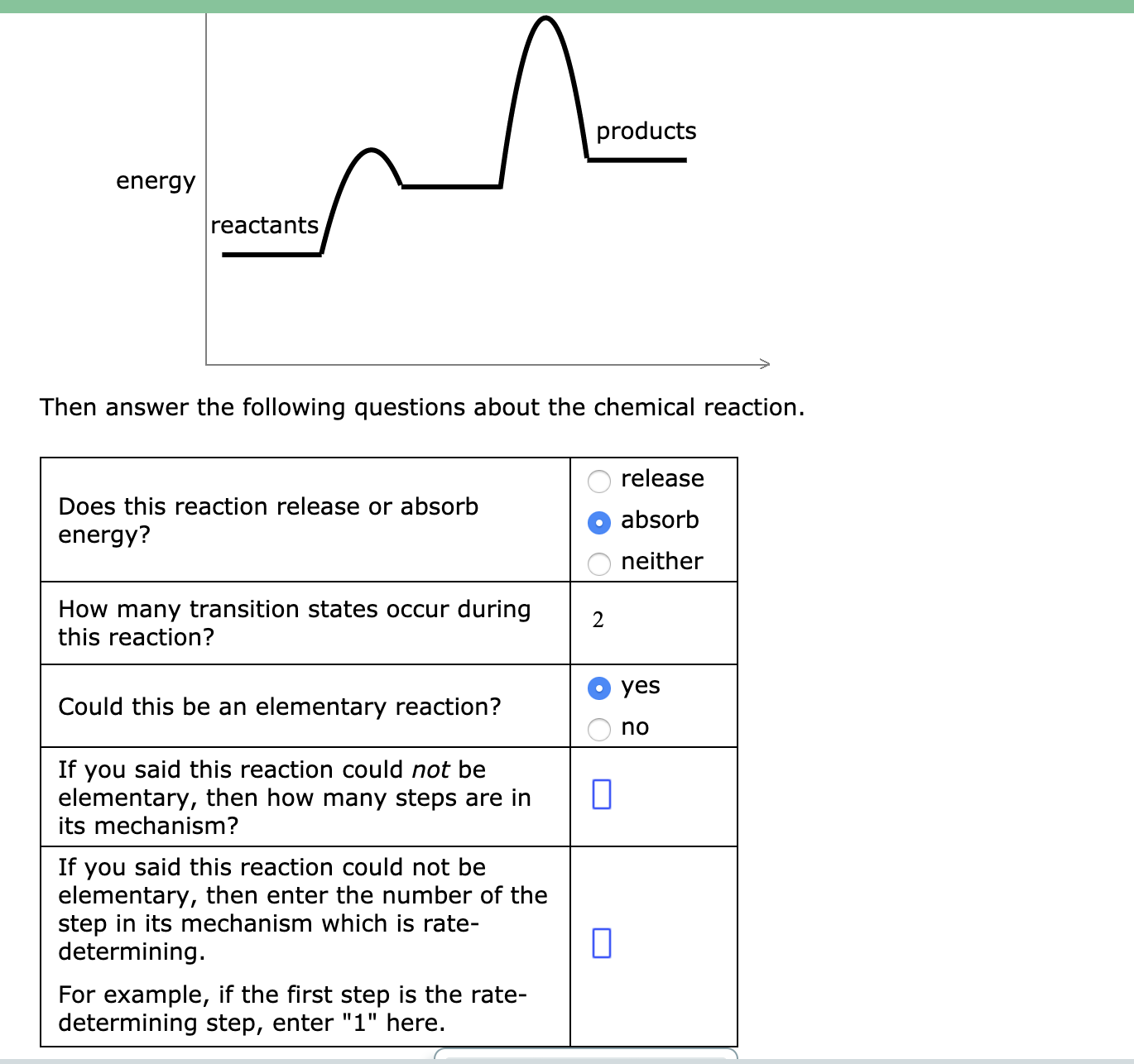










0 Response to "42 chemical reactions that release energy"
Post a Comment