38 examples of specific heat
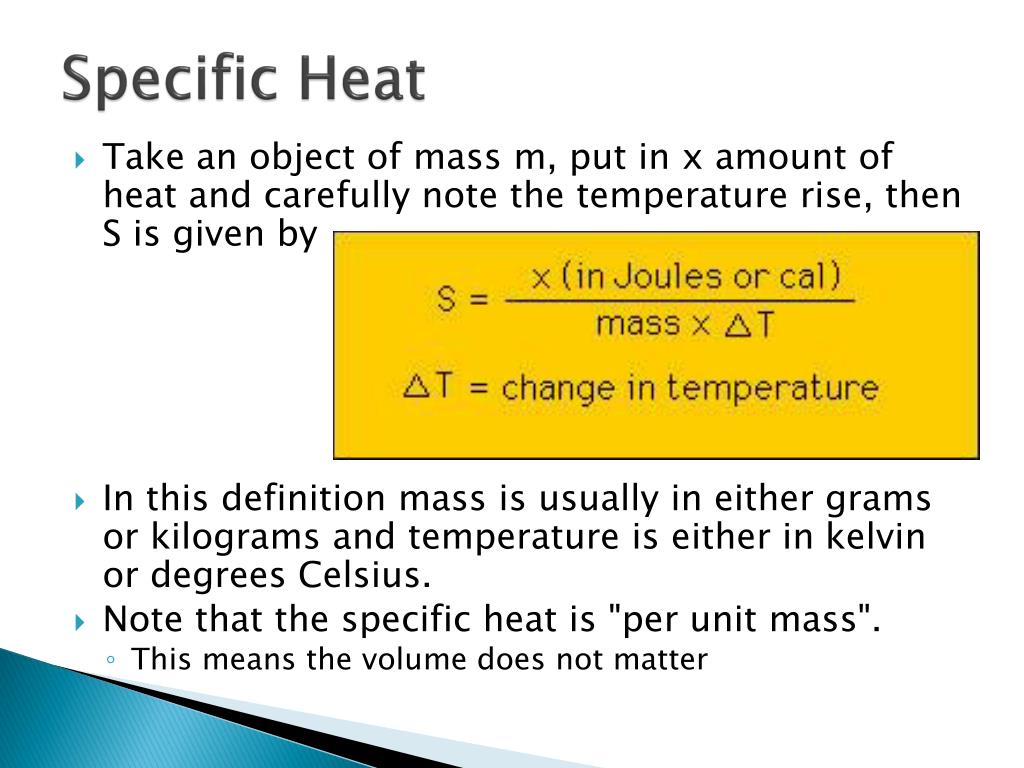
Specific Heat Worked Example Problem - ThoughtCo Specific heat is defined as the amount of heat per unit mass needed to increase the temperature by one degree Celsius (or by 1 Kelvin). Usually, the lowercase letter "c" is used to denote specific heat. The equation is written: Q = mcΔT (you can remember this by thinking "em-cat") What are some examples of specific heat? - Quora Now best example to Specific heat is Water, for water specific heat is 1. real life example of specific heat: water takes more time to heat up and cool down. Take a drum of water and a drum of other liquid Heat up both for same time and with same flame.
Specific Heat Capacity & Water - Formula & Detailed Explanation with ... This value for Cp is actually quite large. This (1 cal/g.deg) is the specific heat of the water as a liquid or specific heat capacity of liquid water. One calorie= 4.184 joules; 1 joule= 1 kg (m)2(s)-2 = 0.239005736 calorie. The specific heat capacity of water vapour at room temperature is also higher than most other materials.

Examples of specific heat
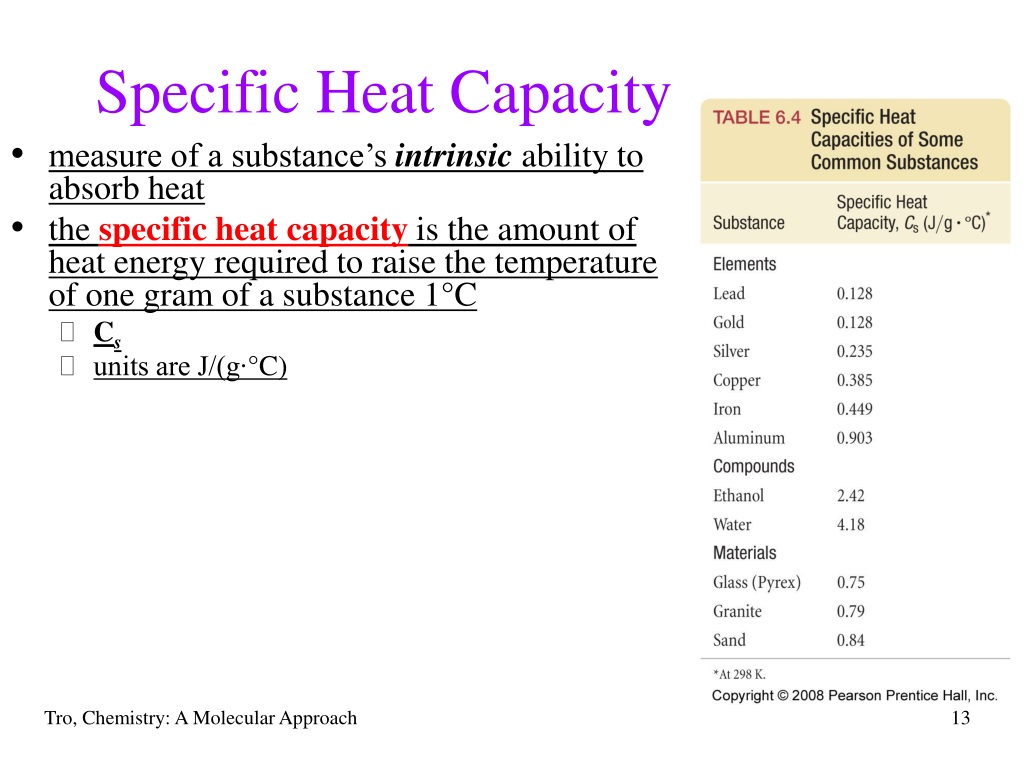
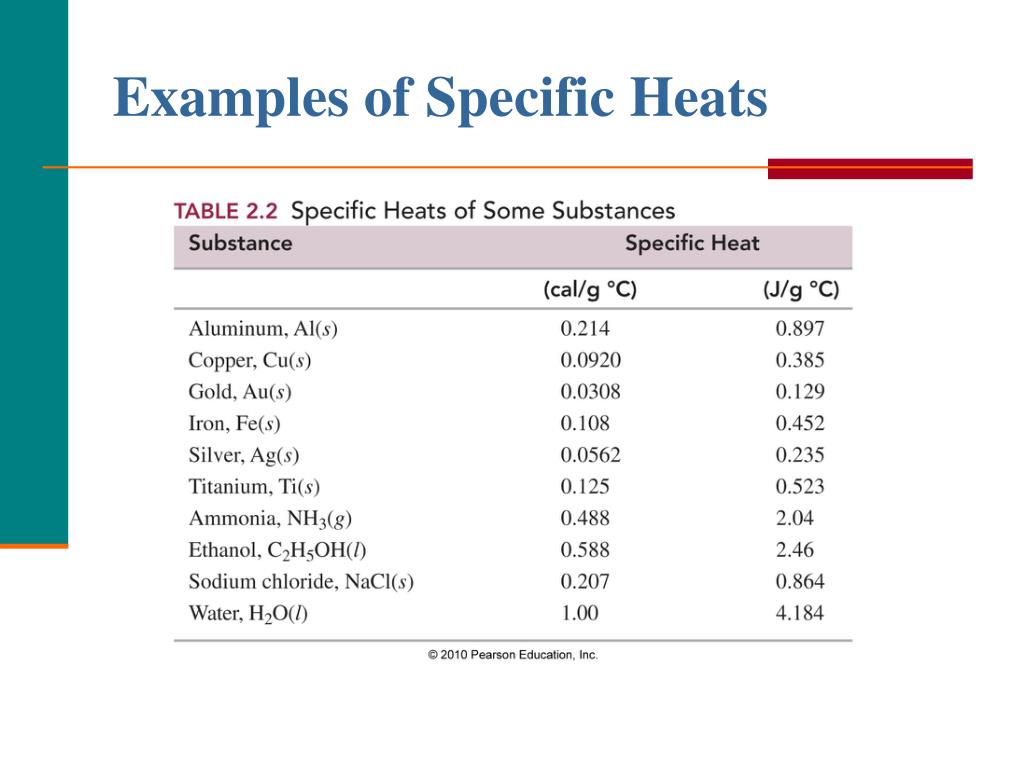
Specific Heat Capacity Definition - ThoughtCo Specific Heat Capacity Examples Water has a specific heat capacity of 4.18 J (or 1 calorie/gram °C). This is a much higher value than that of most other substances, which makes water exceptionally good at regulating temperature. In contrast, copper has a specific heat capacity of 0.39 J. Table of Common Specific Heats and Heat Capacities Specific Heat Formula - Definition, Equations, Examples Solved Examples on Specific Heat Formula Example 1 If the specific heat of gold is 129 . Then what quantity of heat energy is required to raise the temperature of 100 g of gold by 50.0 K? Solution: First of all, write down the things given in the question Mass of the gold = 100 g converting it into kg, we get 0.100 kg. Specific heat = 129 . specific heat | Definition & Facts | Britannica specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one Celsius degree. The units of specific heat are usually calories or joules per gram per Celsius degree. For example, the specific heat of water is 1 calorie (or 4.186 joules) per gram per Celsius degree.
Examples of specific heat. Specific Heat Formula- Explaination,Formula with Solved Examples Specific Heat is the quantity of heat essential to raise the temperature of a unit mass of the substance by one unit. The specific heat capacity is different for various substance. ... Solved Examples. Example 1: Calculate the heat required to raise 0.6 Kg of sand from 30 o C to 90 o C? (Specific Heat of sand = 830 J/Kg o C) Answer: Known: Specific Heat Capacity and its Applications | Definition, Examples ... Specific heat capacity of a substance is defined as the amount of heat energy required to raise the temperature of unit mass of the substance by 1 K. c= mΔtQ Its SI unit is Jkg −1K −1 Note: c= mc where c is the heat capacity. example Measuring specific heat capacity of a solid in calorimeter Specific heat capacity - Wikipedia Specific heat capacity. In thermodynamics, the specific heat capacity (symbol cp) of a substance is the heat capacity of a sample of the substance divided by the mass of the sample, also sometimes referred to as massic heat capacity. Informally, it is the amount of heat that must be added to one unit of mass of the substance in order to cause ... Specific heat Definition & Meaning - Merriam-Webster specific heat: [noun] the heat in calories required to raise the temperature of one gram of a substance one degree Celsius.
Specific Heat Calculator Calculate specific heat as c = Q / (mΔT). In our example, it will be equal to c = -63,000 J / (5 kg * -3 K) = 4,200 J/ (kg·K). This is the typical heat capacity of water. If you have problems with the units, feel free to use our temperature conversion or weight conversion calculators. Heat Energy Examples - Softschools.com Examples of Heat Energy: 1. A cup of fresh coffee is very hot because the water particles are vibrating very rapidly giving the coffee heat energy. 2. A popsicle has particles vibrating very slowly so there is a small amount of heat energy and the popsicle is cold. 3. Specific Heat Capacity (video lessons, examples, step-by-step solutions) The specific heat capacity of water is 4200 J/kg °C. An iron has an aluminium plate with a mass of 1.5kg. Calculate the thermal energy stored in the plate when the temperature rises from 20°C to 200°C. The specific heat capacity of aluminium is 913 J/kg° C. A hot water bottle cools down from 80°C to 20°C, releasing 756000J of thermal energy. Examples of Specific, sensible and latent heat - LORECENTRAL Specific heat also depends on atmospheric pressure. The same substance at a lower atmospheric pressure has a lower specific heat. The examples below are valid for a temperature of 25 degrees and a pressure of 1 atmosphere. Sensible heat The sensible heat is the amount of heat that can receive a body without affecting its molecular structure.
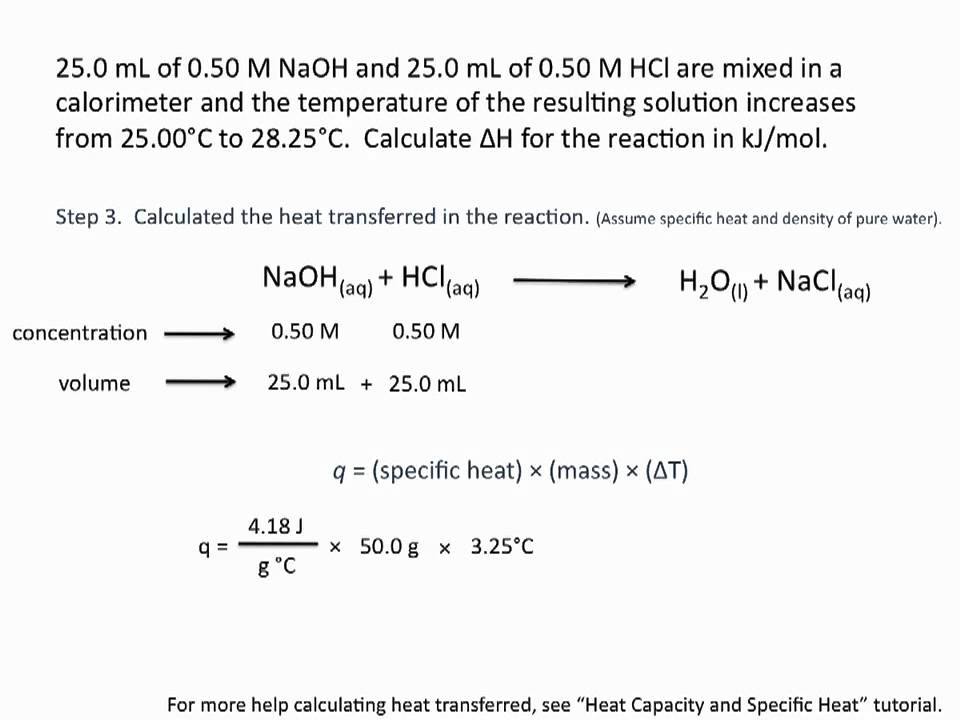
Calorimetry Examples: How to Find Heat and Specific Heat Capacity - YouTube Figure out how to find the heat and specific heat capacity in these two common calorimetry examples. In this video I also go over keywords like heat absorbed... Give 10 examples of specific heat in our daily life...and explain a ... Everything has a specific heat. Specific is the measure of the heat energy required to increase the temperature of 1 kg of a substance by 1 degree Celsius or Kelvin. For example the specific heat of water(l) is 4220mJ/KG x K. oiling the kettle for a nice cup of tea...requires the addition of Specific Heat to the water. Specific heat capacity - Energy and heating - AQA - BBC Some other examples of specific heat capacities are: Material Specific heat capacity (J/kg/°C) ... specific heat capacity (c) is measured in joules per kilogram per degree Celsius (J/kg°C) Specific Heat Formula - Equation, Derivation, Examples and FAQs Specific Heat Examples Now, let us have a look at a few specific heat examples that will help us to understand the concept of specific heat and the specific heat equation or the specific heat formula in a better way. 1) If 968 joules of heat is required to raise the temperature of the 50 g of substance from 3000c to 4000c.
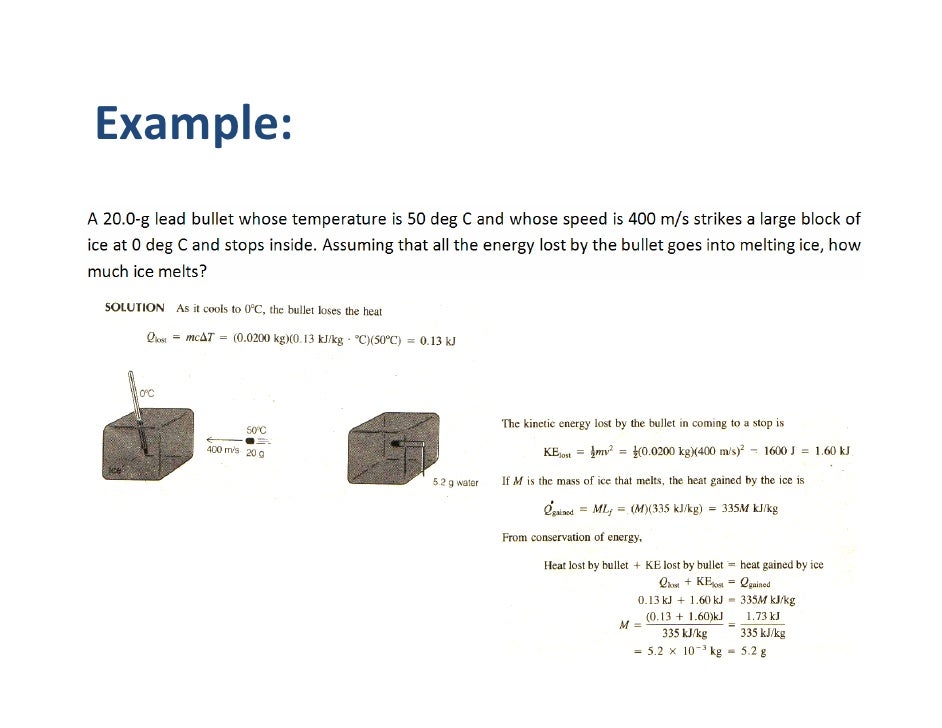
EXAMPLES SPECIFIC HEAT...doc - ASIGNMENT Specific Heat Q The specific heat of lead is 0.129 J/g°C. Solution: First, let's the variables we know. m = 500 grams c = 0.129 J/g°C ΔT = (Tfinal- Tinitial) = (75 °C - 25 °C) = 50 °C Plug these values into the specific heat equation from above.
Specific Heat of Constant Pressure - Heat Capacity and ... - VEDANTU Special heat capacity is measured in J/ (kg °C) or equivalently in J/ (kg K). C=cm or c=C/m is the relationship between the capacity for heat and the specific heat. The mass m, specific heat c, change in temperature ΔT, and heat added (or subtracted) Q are related by the equation: Q=mc Temperature and phase of substances have an effect on ...
What are some real life examples of specific heat with ... - Quora specific heat capacity of water is 4.184 kj/kg-k and that of milk is around 3.6 kj/kg-k , so when your tea contains more of water and less quantity of milk ( which is not a tea you want to drink) , when this hot tea is kept to cool it will be hotter than the tea kept to cool in which water content is less and milk content is more, after same span …
specific heat | Definition & Facts | Britannica specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one Celsius degree. The units of specific heat are usually calories or joules per gram per Celsius degree. For example, the specific heat of water is 1 calorie (or 4.186 joules) per gram per Celsius degree.
Specific Heat Formula - Definition, Equations, Examples Solved Examples on Specific Heat Formula Example 1 If the specific heat of gold is 129 . Then what quantity of heat energy is required to raise the temperature of 100 g of gold by 50.0 K? Solution: First of all, write down the things given in the question Mass of the gold = 100 g converting it into kg, we get 0.100 kg. Specific heat = 129 .
Specific Heat Capacity Definition - ThoughtCo Specific Heat Capacity Examples Water has a specific heat capacity of 4.18 J (or 1 calorie/gram °C). This is a much higher value than that of most other substances, which makes water exceptionally good at regulating temperature. In contrast, copper has a specific heat capacity of 0.39 J. Table of Common Specific Heats and Heat Capacities







0 Response to "38 examples of specific heat"
Post a Comment