39 electrolysis of water lab answers
In the initial discovery of electrolysis, an acidic water solution was used, but nowadays there is a trend towards alkaline electrolytes such as potassium hydroxide (KOH). 2. Theory of water electrolysis The electrolysis of water is considered a well-known principle to produce oxygen and hydrogen gas. In this lab, you will build an electrolytic cell - an apparatus for carrying out electrolysis and use it to produce various gases and Part 1 - Electrolysis of water. In this section, you will use electricity to split apart water molecules! When the timer goes off, unhook the battery. In the meantime, answer.
Electrolysis of water for energy storage is being very actively pursued. Once generated, there are numerous uses for hydrogen in electrical and natural gas grid, mobility, biogas , and fertilizer applications. Due to their higher operating current densities, high-pressure output...
Electrolysis of water lab answers
Some of the water will ionise, that is, turn to hydrogen (H +) and hydroxide (OH –) ions. When the sodium chloride is dissolved in water, the ions forming the ionic solid separate out. This means that there are actually 4 ions present in the solution: H +, OH –, Na + and Cl –. The negative ions are attracted to the positive electrode. The ideal electrolyte of water electrolysis is one that does not appreciably interfere with the electrolysis process. From a purely theoretical standpoint, almost all electrolytes that induce ionisation in water can be used. When these ionic agent... If you do not care about the water being clear, graphite is a good alternative, as it only tends to This is the ideal metal since it doesn't corrode at all under the conditions used for electrolysis. I'm not a chemist and I tried Googling it but unfortunately did not see an answer that was relevant for me.
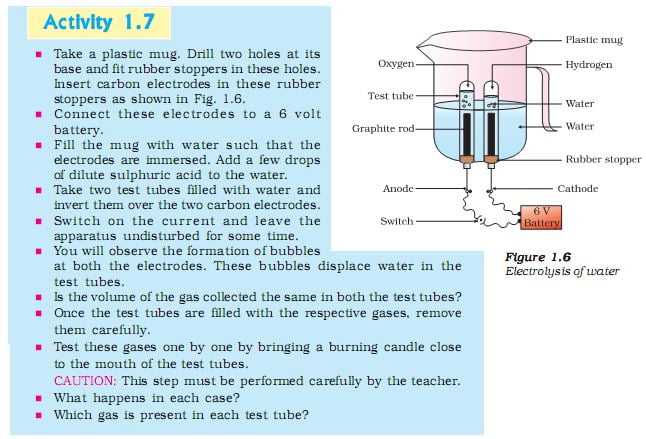
Electrolysis of water lab answers. $\begingroup$ @Mathelogician Electrolysis of water is not an equilibrium process to start with. If you supply current, you'll split water, if you don't you don't. I'm adding another answer, representative of another mechanism, which I think is probably more likely than the one I proposed. It follows a similar... With your own water electrolysis machine you can separate the alkaline and acidic minerals present in tap or well water to make natural alkaline water and acidic ionized water. This water alkalizer works by separating the minerals in your water allowing you to create water from a ph of 3.0 to 12.0 and a -ORP value of +600mV to -800mV. Activity: Electrolysis of Water. NGSS Science and Engineering Practices Answer the following questions based on the gas formed at each electrode. a. How much gas formed at each electrode? b. How do the volumes of the gases compare? c. How might you explain any differences? d. How does... Electrolysis of Water. This lab will go in your lab book. Follow the directions below to complete the lab. Purpose (put into lab book). Conclusion (answer in lab book). Draw a diagram of your lab set-up (exactly as it appears in this lab). Label the positive and negative end of the battery, show the flow of...
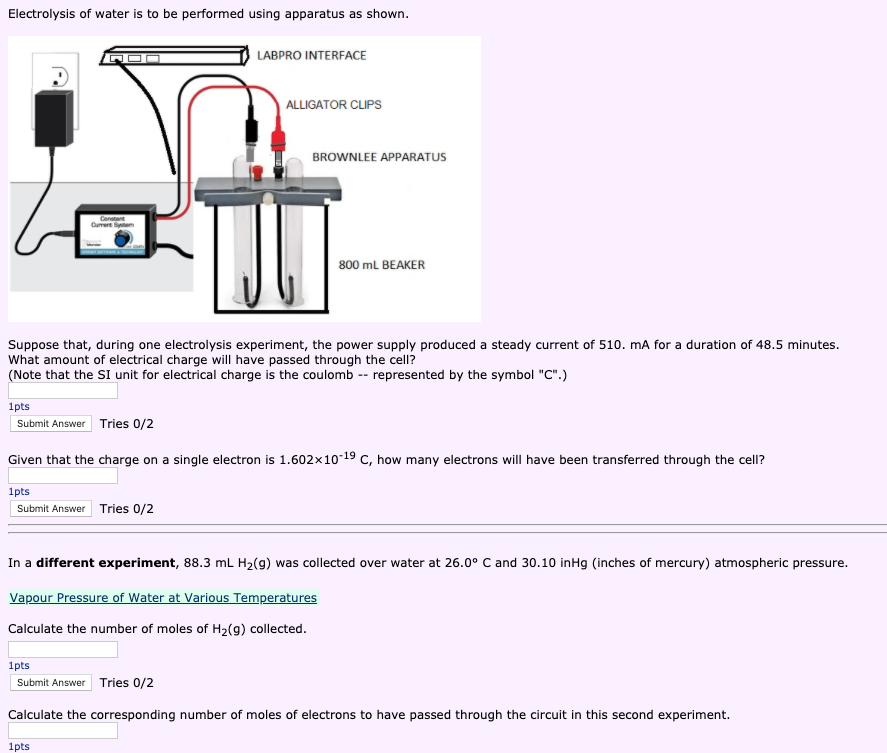
by TA Davis · 2015 · Cited by 31 — Electrolysis of water is a standard chemistry experiment, but the typical laboratory apparatus (e.g., Hoffman cell) is best suited for group ...Article Views: 15957Introduction · Electrolysis in a PDMS Chip · Teaching Electrolysis with Chips Water electrolysis is known more than 130 years already, and different technologies are developed giving power consumption around 3.6 kWh/m3 The studies revealed a few significant differences compared to conventional DC electrolysis of water. New model is established and described, as well... Nov 29, 2021 · Make sure you only use batteries in this kind of experiment rather than plugging anything into a wall socket. The reaction you are going to demonstrate is called electrolysis. When a current passes through salty water it dissociates and creates bonds with both the water (H2O) and the salt (NaCl). Electrolysis of Water Lab - Free download as PDF File (.pdf), Text File (.txt) or read online for free. Electochemistry. Electrolysis of Water. OVERVIEW. During electrolysis, electrical energy is used to cause a nonspontaneous chemical reaction to occur.
Splitting Water: Electrolysis Experiments + Video. Electrolysis is the process by which an electric current is passed through a substance to Electrolysis is used to remove hair, split compounds, and in the manufacturing process to decorate, strengthen, and make metal surfaces more resistant to rust. The solid sodium reacts with liquid water to produce molecular hydrogen gas and the ionic compound sodium hydroxide (a solid in pure form, but readily dissolved in water). Special conditions necessary for a reaction are sometimes designated by writing a word or symbol above or below the equation’s arrow. >>Electrochemistry. >>Electrolytic Cells and Electrolysis. >>Explain electrolysis of water? | Chemist. Question. Electrolysis of water is the decomposition of water (H2 O) into oxygen (O2 ) and hydrogen gas (H2 ) due to an electric current being passed through the water. Background Electrolysis of water is the process by which water is decomposed into oxygen and hydrogen This lab has been taken from Addison Wesley Chemistry Laboratory Manual and modified by Action of electricity on water (electrolysis)- Water can be broken down into its component...
LAB: Electrolysis of Water Electrolysis Apparatus 2H2O 2H2 + O2 Water is a polar molecule meaning that one end (the hydrogen atoms) are positively charged (+) and the other end (oxygen side) is negatively (-) charged. The negative and positive charges on the ends of a battery can be used to...
Alkaline water electrolysis is one of the easiest methods for hydrogen production, offering the advantage of simplicity. The challenges for widespread use of water electrolysis are to reduce energy consumption, cost and maintenance and to increase reliability, durability and safety.
Sep 22, 2021 · Damien has a master's degree in physics and has taught physics lab to college students. An ammeter is a tool for measuring electrical current that got its name from ampere, which is the standard ...
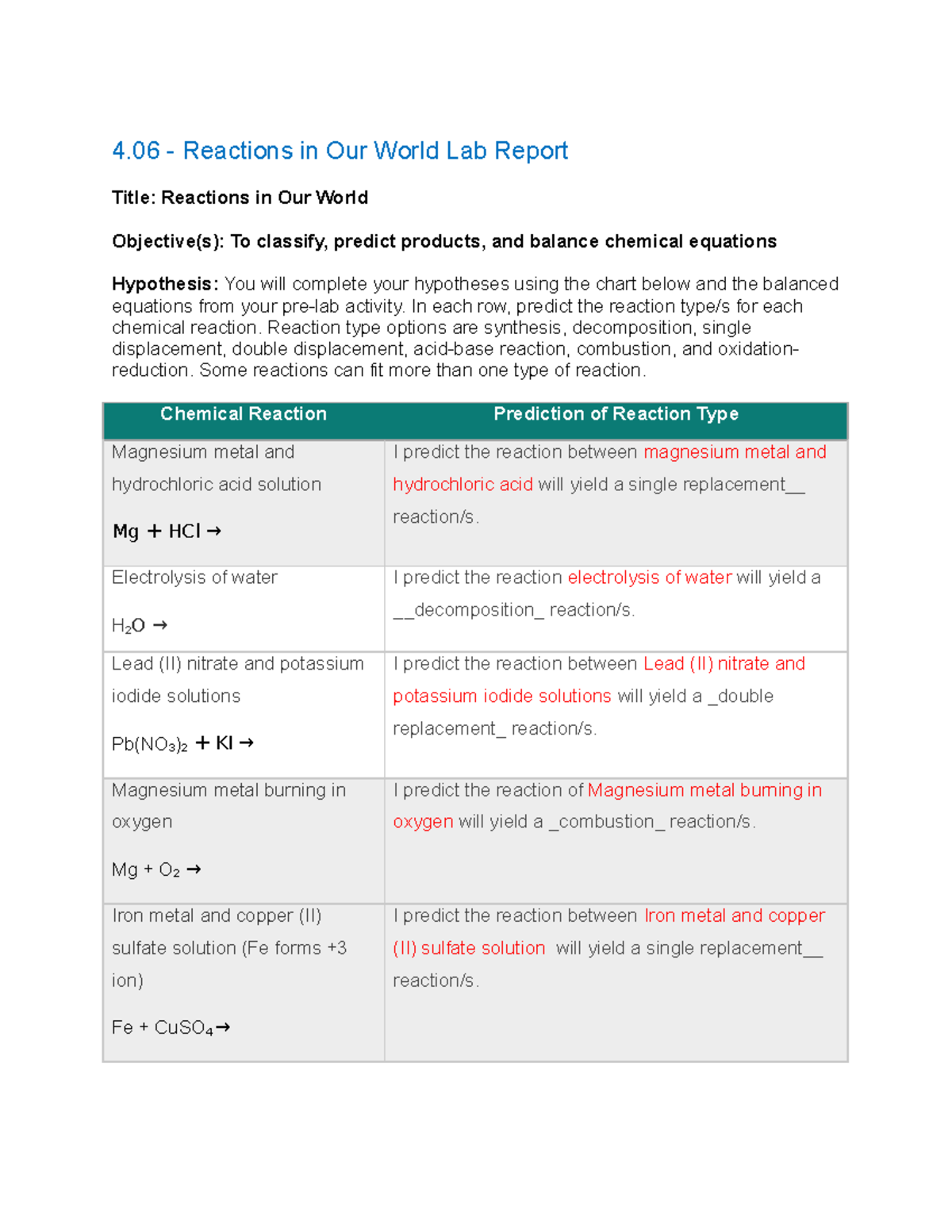
You can just answer letters no need to put explaination but if u want too its okay :)) 4. The electrolysis of water into hydrogen and oxygen is an example of _ reaction. A. Combination C. double replacement B. Decomposition D. single replacement. 5. What scientific law is explained using...
Electrolysis of water is the decomposition of water into oxygen and hydrogen gas due to the passage of an electric current. This technique can be used to make hydrogen gas, a key component of hydrogen fuel, and breathable oxygen gas, or can mix the two into oxyhydrogen - also usable as fuel, though...
Electrolysis of water 2. Thread starter chanller. Start date Sep 1, 2007. I believe the answer to your electrolysis questions is that before you actually go about performing an experiment, you should make yourself thouroughly familiar with the theory - that means completing the chapters on electrochemistry...
Jun 18, 2020 · For example: Measure 22.5 mLs of the stock 5 M solution of NaCl and dilute it with 52.5 mLs of water. Stir to mix. Label the container with both the concentration and the compound: 1.5 M NaCl. Remember, if you are diluting an acid with water, always add the acid to the water.
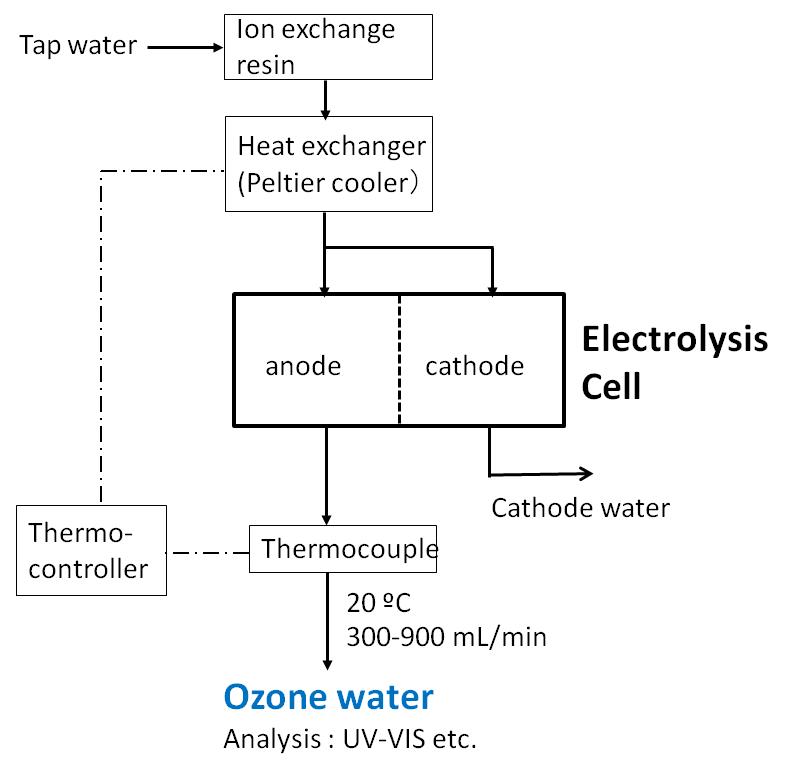
Electrolysis of Water. When electrolysis is performed using a dilute aqueous NaOH solution as an electrolyte, hydrogen gas is generated at the cathode (-), and oxygen gas is generated at the anode (+).
Review and cite WATER ELECTROLYSIS protocol, troubleshooting and other methodology information | Contact experts in WATER ELECTROLYSIS to get answers. Explore the latest questions and answers in Water Electrolysis, and find Water Electrolysis experts.
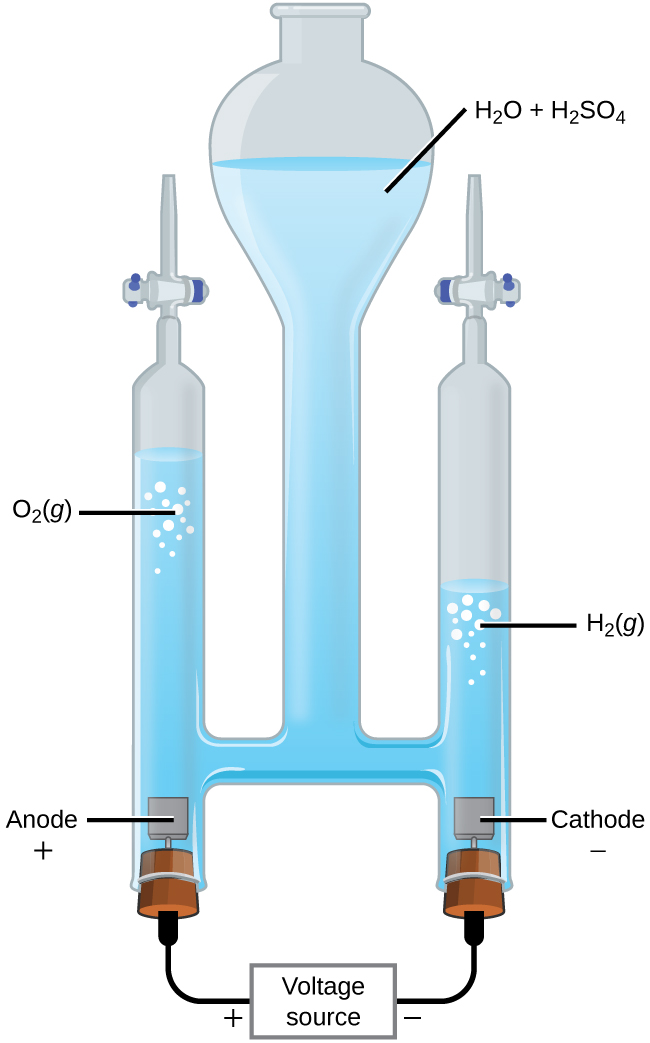
Electrolysis of water. Electrolysing water splits the water molecules (H2O) into hydrogen (H2) and oxygen (O2) molecules according to the This confirms that there are twice as many hydrogen atoms as oxygen atoms in water. At the negative electrode (cathode) a reduction reaction occurs.
Electrolysis of water can be achieved in a simple hands-on project, where electricity from a battery is passed through a cup of water (in practice a saltwater solution All basic lab safety guidelines for your classroom/lab should be followed. Activity o Have the students read the "Background" section (above)...
Electrolysis of water, What is the lowest of electrolite used today in the production of hydrogene from water , using 12 or 24 volts power supply. I think what you may want answered is what is the amount to give the fastest or most economical electrolysis using 12 or 24 volts??
Electrolysis of water results into Hydrogen and oxygen in ration 2:1 by volume. Hydrogen collects at anode as it undergoes ... Inquiry lab and stoichiometry calculations to determine the balanced chemical equation for decomposition of baking soda.
The electrolysis of acidified water. Doc Brown's chemistry revision notes: GCSE chemistry, IGCSE chemistry, O level & ~US grades 9-10 school science courses or equivalent for ~14-16 year old students of chemistry Science AQA GCSE chemistry Edexcel GCSE Chemistry OCR 21st Century...
In this free science fair project idea, kids will conduct an easy electrolysis of water experiment to test solutions of salt, baking soda, tap water, and more.
Electrolysis of Water / Page 1. Procedure (during class) 1. Read the following passage to the students (This passage also appears on the student's. 2. Divide the students into lab groups of 3 - 4 students per group. Give the students an overview of what they will be doing. Then break it down into smaller...
This chemistry video tutorial provides a basic introduction into the electrolysis of water which splits H2O into H2 (hydrogen has) and O2 (oxygen gas).
The electrolysis of water produces hydrogen and oxygen gases. The electrolytic cell consists of a pair of platinum electrodes immersed in water to The electrolysis of water is described. Contributors and Attributions. CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna...
Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas. Hydrogen gas released in this way can be used as hydrogen fuel...
If you do not care about the water being clear, graphite is a good alternative, as it only tends to This is the ideal metal since it doesn't corrode at all under the conditions used for electrolysis. I'm not a chemist and I tried Googling it but unfortunately did not see an answer that was relevant for me.
The ideal electrolyte of water electrolysis is one that does not appreciably interfere with the electrolysis process. From a purely theoretical standpoint, almost all electrolytes that induce ionisation in water can be used. When these ionic agent...
Some of the water will ionise, that is, turn to hydrogen (H +) and hydroxide (OH –) ions. When the sodium chloride is dissolved in water, the ions forming the ionic solid separate out. This means that there are actually 4 ions present in the solution: H +, OH –, Na + and Cl –. The negative ions are attracted to the positive electrode.






























0 Response to "39 electrolysis of water lab answers"
Post a Comment